
Male Infertility
Male Infertility
Last Section Update: 02/2023
Contributor(s): Maureen Williams, ND; Stephen Tapanes, PhD; Carrie Decker, ND, MS
Table of Contents
- Overview
- Structure & Function of the Male Reproductive System
- Causes of Male Infertility
- Risk Factors for Male Infertility
- Nutrients
- Diet & Lifestyle Changes to Support Male Fertility
- Diagnosing Male Infertility
- Male Infertility Treatment
- Living with Male Infertility
- Frequently Asked Questions About Male Infertility
- Update History
- References
1 Overview
Summary and Quick Facts for Male Infertility
- Infertility affects about 15% of all couples in the United States.1
- As many as half of all cases of infertility involve reduced male fertility, thus, it is important for the fertility of both partners to be assessed when a couple is having trouble conceiving.
- The male is solely responsible for about 20% of cases of infertility.1,2 An additional 35% of cases have both male- and female-specific issues.3
- Possible causes of fertility problems problems include testicular problems resulting in abnormal sperm parameters, hormonal conditions, and problems with sperm transport. In many cases, the cause is unknown (ie, the infertility is said to be “idiopathic”).1
- A diagnosis of male infertility, and its subsequent treatment, can be a source of distress for couples.4
- Numerous nutrients, including selenium, zinc, coenzyme Q10, L-carnitine, and omega-3 fatty acids, have a promising research record for improving sperm quality and pregnancy rates.5,6
- Some types of male infertility can be treated.
- For men with untreatable infertility, assisted reproductive technologies or adoption are other options for growing a family.
- Without treatment, about 23% of untreated infertile couples may still conceive within two years and 33% conceive within four years.1
2 Structure & Function of the Male Reproductive System
Understanding the structure and function of key components of the male reproductive system will help provide important context as you learn about male infertility and its treatment.
The male reproductive system is made up of the following structures7,8:
- Testes. The testes (or testicles) are the male gonads, the site of sperm production and maturation. The development of mature sperm from germ cells in the seminiferous tubules of the testes is regulated by specialized testicular cells known as Sertoli cells, which are activated by follicle stimulating hormone (FSH) from the pituitary gland in the brain. Other testicular cells called Leydig cells produce testosterone when stimulated by another pituitary hormone known as luteinizing hormone (LH). Testosterone is necessary for spermatogenesis and also supports male development and maturation.7
- Epididymis. There is an epididymis on the back of each testicle. Epididymides are convoluted tubular structures where sperm undergo final maturation and begin to move out of the testes. The tail end of each epididymis stores sperm until ejaculation occurs.7
- Scrotum. The scrota are the external pouches of skin that house the testes and epididymides.7 Their position outside the body core allows the testes and epididymides to be kept at a lower-than-body temperature, which is optimal for sperm production and storage.9
- Vas deferens and ejaculatory duct. Each epididymis is connected to a long muscular tube known as the vas deferens that transports sperm into the pelvis. The vasa deferentia join structures called seminal vesicles just below the bladder to form the ejaculatory ducts, which empty into the urethra.7
- Seminal vesicles, prostate gland, and bulbourethral glands. The ejaculatory ducts, formed by the fusion of the seminal vesicles and vasa deferentia, carry sperm through the prostate gland and past the bulbourethral (aka Cowper’s) glands. The seminal vesicles, prostate gland, and bulbourethral glands contribute to the production of semen. Semen is a nutrient-rich and slightly alkaline fluid that carries, supports, and protects sperm as they travel through the female reproductive tract, where fertilization can potentially occur.7
- Urethra. The urethra is the passageway inside the penis that can transport either urine or semen.7 When a man ejaculates, a muscular sphincter at the base of the bladder closes so semen does not enter the bladder and urine is not released into the urethra.10
- Penis. The penis is a highly vascularized muscular organ. Sexual stimulation can trigger an increase in blood flow to the penis, causing it to become enlarged and erect.7 Proper erectile function is not necessary for a man to be fertile per se. However, a couple in which the male partner has erectile dysfunction will likely have trouble conceiving because an erection enables the release of semen in close proximity to the cervix, where sperm enter the female uterus.11
3 Causes of Male Infertility
Structural or functional problems affecting any aspect of the male reproductive system can influence male fertility. Testicular problems resulting in abnormal sperm parameters—the vast majority of which are idiopathic (of unknown cause)—are involved in approximately 65–80% of cases of male infertility; hormonal conditions that affect reproductive functioning cause 2–5% of cases; and, problems related to sperm transport are responsible for about 5% of cases. In about 10–20% of men with infertility, sperm and semen parameters are normal and the infertility is unexplained.1
Causes of male fertility problems can be generally categorized as follows4:
- Pre-testicular. This includes problems related to the hypothalamus and pituitary gland, which are responsible for generating hormones to regulate testicular function.
- Testicular. This includes chromosomal (genetic) and non-chromosomal causes of defective sperm production.
- Post-testicular. This includes problems related to the ducts that transport sperm out of the body, as well as conditions that damage sperm after they leave the testes.
- Idiopathic. When the cause of an abnormal semen analysis is unknown, it is considered idiopathic.
Pre-Testicular Causes
Conditions that disrupt normal signaling of the hypothalamus and pituitary gland are pre-testicular causes of male infertility. The hypothalamic-pituitary-gonadal (HPG) axis in men regulates male reproductive function and is comprised of hormone-producing glands in the brain and their target organ, the testes. Gonadotropin releasing hormone (GnRH) from the hypothalamus stimulates the anterior pituitary gland to release gonadotropins—follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH activates Sertoli cells to initiate sperm production and LH promotes testosterone production by Leydig cells.7,12 Activated Sertoli cells also release a hormone called inhibin B that helps regulate the HPG axis through negative feedback by suppressing FSH release from the pituitary gland. Testosterone also exerts negative feedback on the hypothalamic and pituitary glands, helping regulate its production.7
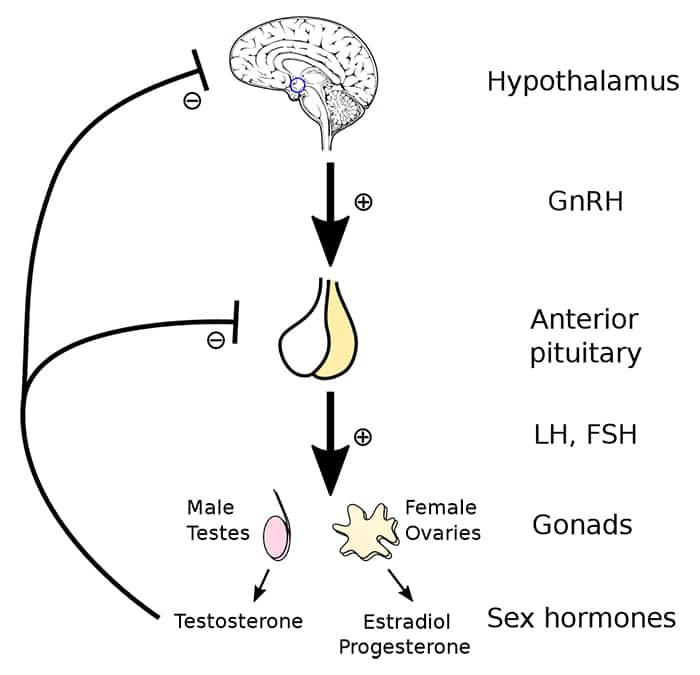
For more information about the HPG axis, see the Male Hormone Restoration protocol.
Hypogonadotropic hypogonadism. Hypogonadism is characterized by low levels of testosterone due to disruption of the HPG axis. In hypogonadotropic hypogonadism, decreased levels of GnRH, FSH, and/or LH result in impaired testicular function, often leading to low energy and infertility in reproductive-aged men.12,13 While certain genetic disorders (eg, Kallmann syndrome) are linked to impaired function of the hypothalamus or pituitary gland from birth, toxic exposures and other environmental factors, both in utero and later in life, are also known to interfere with the activities of the gonadotropic hormones.13-15 This includes exposures to toxins and drugs with endocrine-disrupting effects like bisphenols, opioids, and anabolic steroids (including testosterone).16 Exogenous testosterone, even in men with low endogenous testosterone production, suppresses release of hypothalamic and pituitary hormones needed to stimulate testicular function.17 Disorders involving hypersecretion of testosterone also suppress hypothalamic and pituitary signaling, thereby reducing gonadotropin release and inhibiting sperm production.1 In addition, pituitary tumors associated with low gonadotropin production, such as prolactinomas (which produce high amounts of the hormone prolactin and can reduce production of other pituitary hormones), can cause infertility.1
Testicular Causes
A normal mature sperm consists of a head, midpiece, and tail. The head houses genetic material and is covered by a cap that contains abundant enzymes to aid in fertilization of the egg. The midpiece is rich in mitochondria that produce energy for the tail, which gives the sperm motility.7 Disordered sperm production by the testes can result in low sperm numbers or defects in sperm morphology (size, shape, and structure) that can contribute to infertility. Sperm disorders are by far the most common cause of male infertility.1
Genetic abnormalities. Genetic abnormalities affect about 15% of men with infertility.2 The human genome normally contains two sex chromosomes that determine the sex of an individual: two X chromosomes in women and one X and one Y sex chromosome in men. Abnormalities or damage affecting these chromosomes are a major genetic cause of male infertility. The presence of an extra X chromosome (Klinefelter syndrome) or an extra Y chromosome, the presence of two X but no Y chromosome in a male, and microdeletions or translocations affecting the X or Y chromosome are examples of chromosomal problems that can disrupt sperm production and overall genital and gonad development, resulting in azoospermia (absence of sperm) and infertility.4 In addition, genetic and epigenetic changes to DNA can affect the ability of sperm to fertilize an egg and create a viable embryo.18,19 Advances in genetic sequencing technologies have led to the discovery of nearly 40 genes that may play a contributing role in male infertility due to azoospermia.19
Certain chemical exposures, both in utero and after birth, are known to have toxic genetic and epigenetic effects that can impair fertility.16 For example, bisphenols are a chemical family with well-studied toxic effects on the genome and epigenome (the structural aspect of genetic material which affects how genes are expressed). Bisphenols are organic chemicals used to manufacture plastics, epoxy resins, and cosmetics such as lipsticks, makeup, and nail lacquers. Bisphenols, including bisphenol A (BPA), have been found to negatively impact both male and female fertility.20,21 Exposures to organophosphates, phthalates, natural gas, mercury, radiofrequency electromagnetic radiation, and air pollution are also thought to induce DNA damage that could contribute to male infertility.16
Varicocele. A varicocele is an enlargement of the network of veins within the scrotum. Some men with varicocele do not suffer from infertility, but in other men, varicoceles interfere with sperm formation, movement, or function. In fact, varicocele is present in 35–40% of men with infertility and 15–20% of the general male population.22,23
Historically, varicocele has been thought to cause infertility by reducing oxygenation and increasing oxidative stress in the testicular environment, but more recent evidence also implicates inflammatory and immune mechanisms. For example, higher levels of inflammatory cytokines and antisperm antibodies have been found in semen from infertile men with varicocele than those without varicocele. Varicocele also appears to damage the integrity of the blood-testicular barrier, a barrier that protects testicular cells by selectively regulating the movement of molecules from the blood into the testicular tissue. An impaired blood-testicular barrier may increase the odds of increased immune and inflammatory activity within the testes.22 Further, varicoceles are thought to contribute to male infertility via impairment of testicular temperature regulation.24
Cryptorchidism. Cryptorchidism, or undescended testes, in which one or both testicles fail to descend into the scrotum, is the most common birth defect of male genitalia. Cryptorchidism in a newborn corrects itself by three months of age in about 80% of cases.25 Persistent cryptorchidism impairs testicular function and can cause infertility if not surgically corrected at a young age. Uncorrected cryptorchidism affecting both testes invariably results in infertility and other problems, while approximately 10% of those with one-sided cryptorchidism develop infertility.24
Other testicular problems. Testicular cancer, testicular trauma, testicular torsion (rotation of the testis, which can cut off blood flow), surgeries that affect testicular blood flow, and abnormal testicular development are some other testicular causes of infertility.2 Certain bacterial and viral infections, such as ascending urinary tract infections, sexually transmitted infections, and viral mumps, can cause epididymitis and orchitis (inflammation of the testes) followed by atrophy and permanent testicular dysfunction.26,27 Exposures to endocrine-disrupting chemical toxins such as herbicides and pesticides, compounds from plastics, heavy metals, natural gas and oil, radiofrequency electromagnetic radiation, air pollution, and noise pollution may directly impair testosterone and sperm production in addition to their effects on sperm DNA.15,16,28 Drugs including tobacco, alcohol, marijuana, and opioids can have a negative impact on sperm quantity and quality.15,16
Post-Testicular Causes
Ejaculation projects sperm from the epididymis into the seminal tract, made up of the vas deferens, ejaculatory ducts, and urethra. During this transit, the fluid components of semen are secreted by the seminal vesicles and prostate gland into the ejaculatory duct. Secretions from the bulbourethral glands and numerous small glands along the urethra produce a thick fluid that clears and lubricates the urethra and facilitates the movement of semen into the female reproductive tract.7 Ejaculatory problems, blockages, and environmental conditions that damage sperm after ejaculation constitute post-testicular causes of infertility.
Obstructive azoospermia. Obstructive azoospermia occurs when a blockage in the seminal tract prevents sperm from being released via ejaculation. Congenital defects, tumors, surgeries, and inflammatory diseases are possible causes of such blockages.1
Sexual dysfunction. Male sexual function involves a highly complex interplay between neurological and hormonal signals.29,30 Sexual dysfunction is a common finding among men coping with infertility, and may be caused by psychological, relational, or other issues related to the inability to conceive. It can also be a cause or contributing factor in the infertility itself. Erectile dysfunction, for example, has been reported in as many as 62% of men in infertile couples, and its severity has been correlated with lower semen quality.31
Ejaculatory problems, such as retrograde ejaculation, premature ejaculation, delayed ejaculation (difficulty or inability to achieve ejaculation), and anejaculation (absence of ejaculation), can be underlying factors in male infertility.10 In retrograde ejaculation semen is pushed back into the bladder instead of out through the urethra. Retrograde ejaculation may be the result of weakness in the bladder neck muscles responsible for preventing reverse flow, or high downstream pressure due to a blockage in the urethra. Lower urinary tract surgeries, medications that affect neuromuscular activity, and diabetes are among the conditions known to be associated with retrograde ejaculation.30 Spinal cord injury is the most common cause of delayed ejaculation and anejaculation.10
Immunologic infertility. The blood-testis barrier separates sperm-generating germ cells from the immune system, protecting them from potentially damaging immune reactions. If the blood-testis barrier becomes compromised through events (eg, surgery or infection) or due to vascular abnormalities (eg, varicocele), the development of antisperm antibodies, which target sperm and decrease their motility, can occur.1,22,32 Women may also experience an immune reaction to sperm that results in antisperm antibody production. It has been estimated that 2–3% of women produce antisperm antibodies. The presence of antisperm antibodies in either partner is linked to increased likelihood of infertility.32
4 Risk Factors for Male Infertility
Age. Male fertility decreases with age. Age-related reductions in reproductive hormone levels, testicular function, chromosomal integrity, and sperm health, as well as increased oxidative stress and cumulative damage to sperm DNA, all contribute to reduced fertility.23,33 In one study, compared with 30-year-old men, 50-year-old men had a 22% lower sperm volume, 37% reduced sperm motility, and 18% fewer morphologically normal sperm.34
Tobacco and nicotine. Cigarette smoking is associated with decreased semen volume, sperm count, and sperm motility, as well as worse infertility treatment outcomes. Nicotine has demonstrated damaging effects on sperm health, and other toxins in cigarette smoke have been shown to act as endocrine disruptors in men. Although less is known about the effects of electronic cigarettes on male fertility, animal research suggests they may have negative impacts on male reproductive health.16 Nicotine exposure via chewing tobacco has also been linked to decreased measures of sperm health and function.35
Alcohol use. High alcohol consumption has been shown in multiple studies to be linked with reduced semen volume and sperm quality. Daily alcohol use has been found to have greater negative impacts on hormonal and semen parameters than occasional alcohol use, and heavy alcohol consumption in men has been associated with longer time to pregnancy.36 Studies indicate alcohol impairs sperm development and disrupts male hormone balance.16,36
Cell phone and laptop use. Cell phones and laptop computers connected to wireless internet emit radiofrequency electromagnetic radiation and create thermal effects that may induce oxidative stress and damage DNA. Although a link between cell phone and laptop use and male infertility has not been definitively established, a growing body of evidence suggests high exposure to these devices can diminish sperm quality and increase DNA damage.16,36 Proximity to the testes may be an issue since studies have noted men who carry their mobile phone in a pants pocket have greater sperm DNA damage than those who carry their phone in a shirt pocket. Similarly, preclinical research suggests placement of a laptop on the lap may be more detrimental than other placements. It is unclear whether these effects are due to radiation, heating, or both.36
Obesity. Infertility is more common in obese men than those with normal weight. The risk of oligozoospermia (low sperm count, also called oligospermia) or azoospermia rises with increasing severity of overweight and obesity. Obesity disrupts the HPG axis and reproductive hormone balance and raises oxidative stress levels, resulting in reduced sperm concentration, motility, and viability, as well as increased numbers of abnormal sperm.23,36,43 It has also been proposed that increased scrotal fat may impair sperm development through thermal effects.36
Diabetes. Both type 1 and type 2 diabetes have been linked to male infertility. A number of underlying mechanisms have been proposed, including increased oxidative stress, disruption of normal hormonal and neurological signaling, impaired cellular and mitochondrial processes, formation of abnormal proteins, and DNA damage.44-46 High blood glucose levels trigger the production of large amounts of advanced glycation end-products (AGEs), proteins and lipids damaged by spontaneous reactions with glucose.47 AGEs increase oxidative stress and inflammation, cause widespread cellular dysfunction, and may damage signaling pathways related to reproductive function.47,48
Sleep apnea. Sleep apnea, a condition where breathing stops and restarts intermittently throughout sleep, has been associated with infertility in both men and women.268-270 Men with obstructive sleep apnea appear to have diminished erectile function, testosterone levels, and semen quality compared to men without sleep apnea.271 Sleep apnea is also closely correlated with obesity, and obesity is associated with increased DNA damage in sperm and male infertility.272 Moreover, sleep apnea causes irregular periods of hypoxia (decreased oxygen levels), which may increase testicular oxidative stress, alter sperm morphology, and decrease sperm concentration and motility.273,274 Adherence to sleep apnea treatment by continuous positive airway pressure (CPAP) may help improve some aspects of fertility.268,271,275
Other chronic systemic disorders:
- Thyroid disorders. Thyroid hormones participate in regulating testicular development and function, and thyroid disorders have been linked to male infertility. Both hyperthyroidism and hypothyroidism are associated with increased percentage of abnormal sperm and decreased sperm concentration, sperm motility, and semen volume, possibly due in part to increased oxidative stress.49
- Cystic fibrosis. Cystic fibrosis is a genetic disorder characterized by chronic lung inflammation and infection, pancreatic enzyme insufficiency, and male infertility.50 Almost all men with cystic fibrosis have a birth defect marked by absence of the vas deferens on both sides; thus, it is impossible for sperm to leave the epididymis. In addition, men without full cystic fibrosis but who harbor mutations in the related gene (known as CFTR) are prone to low sperm counts and obstruction of the epididymis and ejaculatory duct.51
- Cancer. While testicular cancer directly impacts testicular function, other cancers and cancer therapies increase the risk of male infertility.4,52 In addition, cancer and infertility are linked by common triggers: conditions causing high oxidative stress levels and a high degree of DNA damage.53
Interestingly, studies suggest male infertility (except in cases that can be attributed to obstruction) may be an early sign of cancer, other impending disease, and death. A study using data from men in the United States found infertility was associated with an increased risk of testicular and other cancers.54 Another study found men with azoospermia had a more than two-fold increased risk of any cancer compared with men without azoospermia.55 A systematic review and meta-analysis of eight cohort studies totaling 168,327 men with infertility found a 43% increase in developing any type of cancer, 91% increased risk of testicular cancer, 48% increased risk of prostate cancer, and a 31% increased risk of melanoma compared to those without infertility.56 Azoospermia in particular has been correlated with increased risk of declining general health and death.57,58 Accumulated DNA damage and cellular senescence are thought to be a common underlying mechanism linking infertility, cancer, cardiovascular disease, and other chronic diseases.18,59 Men with a history of infertility should consult their healthcare provider and follow general cancer screening recommendations appropriate for their age and health status.
Nutritional deficiencies. Zinc is needed for normal sperm production and function and is concentrated in the prostate gland and semen. Zinc deficiency is associated with low testosterone levels, low sperm motility and viability, and oligo-spermia and azoospermia. Selenium is needed for balancing oxidative and reduction reactions and regulating oxidative stress. Both selenium deficiency and excess increase the risk of infertility due to abnormal sperm motility and viability.60 Blood tests for zinc and selenium can help rule out deficiency.
Stress. Psychological stress is thought to be a risk factor for male infertility.61 Unfortunately, infertility and its treatments can themselves be a source of stress, potentially adding to problems such as erectile dysfunction and retrograde ejaculation.62 Some, but not all, studies have found correlations between high stress levels and reduced semen quality.61,62
Tight clothing and sitting. Decades of research indicate restrictive clothing increases scrotal temperature and may suppress sperm production.9,63 An observational study that included 656 male partners of infertile couples found those who reported regularly wearing boxer-style underwear had 25% higher sperm concentrations, 17% higher sperm counts, and 14% lower FSH levels compared with men who regularly wore tighter styles of underwear.64 Another study in 1,311 men found those who regularly wore tight underwear were more likely to have abnormal semen analyses than those who did not.65 However, an observational study that followed 501 couples attempting pregnancy for 12 months found neither men’s daytime nor nighttime underwear choice had an impact on time to conception or risk of infertility.66
Prolonged sitting is another factor that can affect scrotal temperature and may affect fertility in men with sedentary occupations such as truck driving.9 Even more typical amounts of sitting may impair sperm quality: a study in 254 men found those who routinely sat during 50% or more of their time at work had substantially more sperm DNA damage compared with men who sat less than 50% of the time at work.67 Sitting with legs crossed appears to have the greatest warming effect on the scrota.68
Medications and recreational drugs. Drug families known to disrupt HPG axis signaling and/or decrease fertility by other mechanisms include23:
- Chemotherapy drugs (such as cisplatin, doxorubicin (Adriamycin, among others), 5-fluorouricil (5-FU), cyclophosphamide)52
- Psychiatric medications23
- Corticosteroids and other immune suppressants23,69
- Some blood pressure-lowering drugs including calcium channel blockers, angiotensin receptor blockers (ARBs), and angiotensin-converting enzyme inhibitors (ACEi).23,69
- Alpha-blockers23
- 5-alpha reductase inhibitors23
- Testosterone and other anabolic steroids17,23,69
- Opioids69
- Cannabis70,71
- Growth hormone-releasing hormone analogs69
5 Nutrients
The treatment approach to male infertility is guided by its cause. In many cases involving sperm abnormalities, the underlying cause is unknown, or is idiopathic. A number of nutrient and herbal therapies have demonstrated potential benefits in this type of idiopathic male infertility and are detailed below. Except where otherwise indicated, the clinical trials described in the following section involved men with idiopathic infertility.
L-carnitine
L-carnitine is an amino acid that can be obtained in the diet and made in the body. It is a cofactor in the metabolism of fatty acids and its derivative acetyl-L-carnitine helps regulate carbohydrate, protein, and fatty acid metabolism and cellular energy production. Interestingly, although 98% of the body’s carnitine is stored in the skeletal muscles and heart, carnitine is also selectively concentrated in the male reproductive tract, particularly the epididymis, where it appears to play a critical role in sperm energy production and maturation.76
Multiple clinical trials have found supplementing with L-carnitine and/or acetyl-L-carnitine, at doses of 1–4 grams per day, can improve sperm characteristics in men with infertility.76 In one trial, 240 men with a history of infertility were given 1 gram of L-carnitine three times daily, 100 mg vitamin E three times daily, or both for three months. Those receiving L-carnitine had improved sperm motility and enzymatic activity, but vitamin E had little impact on sperm characteristics.77 In a controlled trial in 262 men with infertility related to poor sperm number, motility, and morphology, a combination of 1 gram of L-carnitine twice daily plus 20 mg CoQ10 three times daily resulted in greater improvement in sperm motility and a higher pregnancy rate than either supplement alone or placebo.78 A meta-analysis of findings from seven randomized controlled trials with a total of 693 participants found combined treatment with L-carnitine and acetyl-L-carnitine together improved sperm motility, increased the number of forward-moving and generally moving sperm, and increased pregnancy rate for men affected by infertility.79
Multi-nutrient supplements containing carnitine have also demonstrated effectiveness at improving sperm parameters and are detailed in the “Nutrient/Antioxidant Combinations” section.
Folic Acid
Folate (vitamin B9) is a cofactor in amino acid metabolism reactions (aka methylation reactions) and helps regulate DNA synthesis and gene expression. Folate also has free radical-scavenging ability, and higher intake has been associated with less sperm DNA fragmentation.41
In a randomized controlled trial in 162 couples with male infertility undergoing in vitro fertilization, those given 15 mg folic acid per day for three months had better outcomes than those given placebo: 44.1% of couples receiving folic acid were able to conceive and 35.6% had a viable pregnancy detectable on ultrasound at seven weeks of gestation compared with 22.4% and 20.4%, respectively, in those receiving placebo.80 Clinical trials using lower doses of folic acid (400 mcg–5 mg [680‒8,500 mcg DFE]) along with other supplements such as zinc, vitamin E, and selenium have not been able to consistently demonstrate benefits on semen parameters or pregnancy rates.81,82
A meta-analysis of 15 studies with a total of 7,466 men found genetic mutations (commonly known as single nucleotide polymorphisms or SNPs [pronounced “snips”]) affecting the methylenetetrahydrofolate reductase (MTHFR) gene that regulates folate metabolism were associated with an increased risk of male infertility.83 One study found these polymorphisms were more common in 167 men with abnormal sperm parameters compared with 78 men with normal sperm. Levels of homocysteine (an amino acid that requires folate to be broken down) were higher in those with male infertility. In addition, folic acid therapy, at 400 mcg (680 mcg DFE) twice daily for three months, increased sperm concentrations more in those with the MTHFR 677 TT genotype (version of the gene) than those with the more prevalent MTHFR 677 CT or MTHFR 677 CC genotypes.84 Another study found higher intakes of folate and vitamin B12 were both associated with lower homocysteine levels and better sperm parameters in men with MTHFR polymorphisms.85 A randomized controlled trial in 769 infertile men with low sperm numbers and MTHFR polymorphisms found, in those with the MTHFR 677 TT genotype, 800 mcg (1,360 mcg DFE) folic acid daily for three months improved semen parameters and sperm fragmentation. Folic acid therapy also resulted in higher pregnancy and live birth rates in men with the MTHFR 677 TT genotype, but this effect did not reach statistical significance.86
Some evidence suggests individuals with MTHFR polymorphisms may need higher doses of folic acid to experience therapeutic benefits and may respond better to supplementation with 5-methyltetrahydrofolate (5-MTHF), the active form of this B vitamin.87
Coenzyme Q10
Coenzyme Q10 (CoQ10) is an essential cofactor for mitochondrial energy production that also has free radical-scavenging ability.88 Some research suggests CoQ10 may help reverse testosterone suppression by endocrine-disrupting toxins.89 A number of clinical trials have reported CoQ10 supplementation has beneficial effects on semen quality and couples’ pregnancy rates when used by men with infertility.88 One meta-analysis of three randomized controlled trials with a total of 296 participants found CoQ10 supplementation resulted in global improvement in sperm parameters.90 A quantitative meta-analysis of trials indicated 3‒6 months of treatment with CoQ10 had its greatest benefit on sperm motility, with smaller effects on sperm count, morphology, and ejaculate volume.91
In a placebo-controlled trial in 228 men with infertility, 200 mg CoQ10 daily for 26 weeks resulted in greater improvements in sperm concentration, motility, and morphology, as well as a reduction in FSH and increase in inhibin B levels.92 Similarly, 300 mg CoQ10 per day for 26 weeks improved sperm parameters, and lowered FSH and LH levels in a placebo-controlled trial in 212 men with infertility.93 A randomized placebo-controlled trial in 50 men with abnormal sperm number, motility, and morphology found 200 mg CoQ10 daily for three months improved sperm parameters and decreased oxidative stress in the seminal fluid.94 As previously described, a controlled trial found 20 mg CoQ10 three times daily, when combined with 1 gram L-carnitine twice daily, resulted in a higher pregnancy rate than either supplement alone or placebo in 262 men with infertility and abnormal semen analyses.78 A report from an uncontrolled randomized trial involving men with poor sperm count, motility, and morphology indicated a group of 35 participants given 200 mg CoQ10 daily for three months had improvements in all aspects of semen quality.95
Myo-inositol
Inositol is a family of isomers (compounds with the same chemical composition but structured differently) found in cells of plants and animals. Myo-inositol is the most abundant inositol isomer occurring in nature and the body, and levels in sperm are particularly high.42,96 Lower myo-inositol levels in semen have been correlated with reduced sperm numbers and motility,97 and myo-inositol supplementation by men has been reported to enhance sperm quality and fertility.96 In a controlled trial that included 37 couples with male infertility, incubating sperm in a myo-inositol-enriched medium prior to artificial insemination (intrauterine) was shown to improve sperm motility and increase the chance of pregnancy.98 Interestingly, the use of vaginal suppositories containing myo-inositol by women in infertile couples has also been reported to increase sperm motility and enhance pregnancy rates relative to placebo.99,100
In a controlled trial that enrolled 62 men, 4 grams myo-inositol plus 400 mcg (680 mcg DFE) folic acid daily for two months increased sperm concentration and motility in those with sperm abnormalities and infertility as well as those with normal sperm and fertility.101 In a placebo-controlled trial including 194 men with infertility, 2 grams myo-inositol plus 200 mcg (340 mcg DFE) folic acid twice daily for three months improved sperm parameters, reduced LH and FSH levels, and increased inhibin B levels.102 Multiple studies have investigated the combination of myo-inositol with other nutrients and are discussed in the “Nutrient/Antioxidant Combination” section.
Zinc
Zinc, a trace element needed for normal male reproductive development, is also essential for sperm production, maturation, and function, and is secreted into semen by the prostate gland because of its important role in ejaculation, sperm release, and fertilization.103 Zinc is a critical part of antioxidant defenses, and deficiency raises oxidative stress and is associated with low testosterone levels, low sperm motility and viability, and oligo- and azoospermia.60,104 A meta-analysis of 20 studies with more than 2,400 participants found zinc levels in the seminal fluid were lower in men with than without infertility. It further showed that zinc supplementation resulted in increased semen volume, sperm motility, and percentage of sperm with normal morphology.105 Another meta-analysis linked low seminal zinc specifically with decreased sperm motility.106,107
In a controlled trial in men with reduced sperm motility, 250 mg zinc sulfate (providing about 58 mg of elemental zinc) twice daily for three months increased sperm number, motility, and fertilization capacity, and reduced the incidence of antisperm antibodies.108 Other clinical trials examining the effect of zinc in men with infertility have used a combination of zinc plus folic acid. A meta-analysis of six randomized controlled trials found zinc plus folate therapy increased sperm concentration and improved sperm morphology, but did not impact sperm motility or hormone levels.109 In a controlled trial in 112 men who had undergone surgery for varicocele, 66 mg zinc plus 5 mg (8,500 mcg DFE) folic acid per day for six months improved sperm parameters and varicocele outcomes more than either alone or placebo.110 However, in one large placebo-controlled trial in 2,370 men with infertility, treatment with 30 mg of elemental zinc plus 5 mg (8,500 mcg DFE) folic acid daily for six months increased DNA fragmentation and did not lead to improvements in sperm parameters or pregnancy rate.82
It is important to note that long-term high-dose zinc supplementation (such as >50 mg daily for >4 weeks) can cause toxicity, mainly due to copper deficiency.111 Consultation with a healthcare provider with nutritional expertise is advised prior to beginning high-dose zinc therapy. Copper supplementation (usually around 2 mg) should be added to any regimen that includes more than 50 mg supplemental zinc daily for more than four weeks.
Selenium
Selenium is needed for regulating oxidative stress, and both deficiency and excess increase the risk of infertility due to abnormal sperm motility and viability.60 A meta-analysis of three randomized controlled trials found selenium supplementation improved sperm concentration, motility, and morphology (size, shape, and structure).5 In one trial, 69 men with reduced sperm motility were given either 100 mcg selenium alone; 100 mcg selenium plus 1 mg vitamin A, 10 mg vitamin C, and 15 mg vitamin E; or placebo daily for three months. Those who received selenium alone or with other vitamins had increased sperm motility and were more likely to achieve pregnancy compared with placebo.112 Three months of treatment with 200 mcg selenium daily was found to improve all sperm parameters in a group of 35 men with poor sperm number, motility, and morphology in an uncontrolled trial.95 However, another trial in 42 men found 300 mcg selenium per day alone for 48 weeks had no effect on sperm count, motility, morphology, or hormone levels.113
N-acetylcysteine
N-acetylcysteine (NAC), a free radical-quenching compound made from the sulfur-containing amino acid cysteine, has been shown to reduce inflammation and oxidative stress, stabilize proteins and DNA, promote mitochondrial resilience, support antiviral immune activity, and promote vascular health.114 A meta-analysis of three randomized controlled trials with a total of 431 men with infertility found NAC supplementation improved sperm numbers, motility, and morphology, and increased ejaculate volume.115 One placebo-controlled trial in 120 men with infertility found 600 mg NAC daily for three months improved semen volume and viscosity, sperm concentration, and antioxidant status.116 As previously noted, in a randomized controlled trial in 468 men with infertility and sperm abnormalities, 600 mg NAC in combination with 200 mcg selenium daily improved semen quality, increased testosterone, LH, and inhibin B levels, and decreased FSH levels after 30 weeks.117 In an uncontrolled trial that included 50 men with poor sperm parameters, 600 mg NAC daily for three months led to improved sperm numbers, motility, and morphology, as well as increased testosterone and LH and decreased FSH levels.118
Lycopene
Lycopene is a free radical-quenching carotenoid pigment found in tomatoes and some other orange and red vegetables and fruits. An observational study indicated higher carotenoid intake was associated with greater sperm motility, and lycopene intake specifically was correlated with better sperm morphology.119 In addition, higher blood levels of lycopene and other carotenoids have been correlated with less sperm DNA fragmentation.120 A clinical trial that enrolled 44 couples being treated for male infertility found lycopene, at a dose of 10 mg twice daily for three months, protected omega-3 fatty acids from oxidative damage, thereby improving the balance of fatty acids in sperm membranes, and resulted in higher spontaneous and assisted pregnancy rates.121
In an uncontrolled trial in 30 men with infertility, the majority of participants experienced improvement in at least one sperm parameter and pregnancy occurred for six participating couples after taking 2 mg lycopene twice daily for three months. The best results were seen in those with milder baseline sperm abnormalities.122 In a randomized controlled trial in 44 men with infertility, 25 mg lycopene daily for 12 weeks led to greater improvements in sperm count, concentration, motility, and morphology, as well as increased semen volume and decreased semen oxidative stress, compared with placebo.123 A trial in 44 men with low sperm concentration and/or motility found those who consumed one can of tomato juice daily, providing 30 mg of lycopene, had increased sperm motility after 12 weeks compared with those who did not take tomato juice.124 In 60 men with infertility participating in a placebo-controlled trial, 14 mg LactoLycopene (a readily absorbed form of lycopene) daily for 12 weeks improved sperm motility and morphology.125
Omega-3 Fatty Acids
Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), help modulate oxidative stress levels, immune function, and inflammation. The fatty acid composition of sperm cell membranes has long been recognized as a factor in sperm function, and DHA in particular appears to be preferentially concentrated in sperm membranes.126 Several studies have noted reduced concentrations of DHA and total omega-3 fatty acids in sperm membranes were correlated with low sperm quantity and quality, as well as varicocele-related and idiopathic male infertility.127-130
Findings from clinical trials indicate supplementation with omega-3 fatty acids may improve sperm parameters in male infertility patients.131,132 In one trial, 211 men with infertility were given 1,120 mg EPA plus 720 mg DHA daily or placebo. After 32 weeks, men in the omega-3 fatty acid group had higher sperm number and concentration, increased sperm motility, and improved sperm morphology.133 Another randomized controlled trial in 57 subjects undergoing evaluation for male infertility found taking an oil providing 990 mg DHA and 135 mg EPA daily for 10 weeks decreased sperm DNA fragmentation and increased seminal antioxidant status more than a sunflower oil placebo, but did not affect other sperm parameters.134 In a trial in 109 men with infertility, 350 mg DHA three times daily for 90 days improved sperm motility, viability, and morphology and decreased sperm DNA fragmentation compared with placebo.135
Not all trials have yielded positive findings. A small randomized controlled trial in 28 men with low sperm motility found neither 400 mg nor 800 mg DHA daily for three months (90 days or about 13 weeks) affected sperm concentration or motility compared with a corn and soy oil placebo.136 In another trial in 180 men with low sperm motility, 465 mg DHA plus 600 IU vitamin E for 12 weeks improved sperm motility as well as sperm number and concentration, but neither supplement alone led to improvements compared with placebo.137
Vitamin E
Vitamin E, a family of compounds that includes tocopherols and tocotrienols, has the ability to end free radical chain reactions, in which one free radical leads to generation of another, and protects lipids, including those in cell membranes, from oxidative damage. Preclinical evidence points to a protective effect on sperm characteristics and a possible role as a therapeutic agent for male infertility.138 In one study, sperm in semen samples were less susceptible to oxidative damage when exposed to a vitamin E-enriched environment.139
Clinical trials exploring the use of vitamin E to treat male infertility suggest possible benefits, especially when used in conjunction with other therapies. A controlled trial in 90 men with low sperm numbers and motility found 400 mg vitamin E daily enhanced the benefits of clomiphene citrate (Serophene, Clomid) on sperm parameters after six months.140 Vitamin E therapy, at 100 mg three times daily for three months, was found to enhance the effects of tamoxifen (Novaldex, Soltamox) therapy in a controlled trial in 64 men with low sperm numbers, and increase the effects of L-carnitine treatment in a controlled trial in 42 men with low sperm motility.141 In an uncontrolled trial in 690 men diagnosed with infertility and found to have low sperm motility and high sperm DNA fragmentation, 14 weeks of supplementation with a combination of 400 IU vitamin E plus 200 mcg selenium daily improved sperm motility, morphology, or both, and resulted in a 10.8% pregnancy rate after 14 weeks.142 In a placebo-controlled trial in 60 men with low sperm motility and increased DNA fragmentation, 400 IU vitamin E plus 200 mcg selenium for three months improved sperm motility and viability.143 However, in a randomized controlled trial in 45 men who underwent surgical treatment for varicocele, additional benefits were not seen with the addition of vitamin E.144
Ashwagandha
Ashwagandha (Withanea somnifera) is a plant used in traditional Ayurvedic herbal medicine to treat a wide array of ailments, including sexual dysfunction and infertility.145,146 Multiple clinical trials indicate ashwagandha may have positive effects on semen parameters, such as semen volume, sperm concentration, and sperm motility, and may help reduce oxidative stress, normalize male reproductive hormones, and increase pregnancy rates in couples being treated for male infertility.147,148 In one placebo-controlled trial in 46 men with low sperm numbers, those who received ashwagandha root extract (standardized to contain at least 5% withanolides), at 225 mg three times daily for 90 days, had a 167% increase in sperm number, 57% increase in sperm motility, and 53% increase in semen volume, while those who received placebo had little change in these parameters. In addition, testosterone and LH levels increased significantly in the ashwagandha-treated men.149 In an uncontrolled trial, 75 men being evaluated for infertility were given 5 grams of ashwagandha root powder daily; after three months, sperm count and motility improved, testosterone and LH levels increased, and FSH and prolactin levels decreased.150 An uncontrolled trial in 60 men with infertility, despite having normal sperm counts, found 5 grams of ashwagandha root powder daily for 90 days reduced levels of markers of psychological stress, decreased semen oxidative stress, and resulted in a 14% pregnancy rate.151
Ginseng
Various types of ginseng, including Panax ginseng (Asian or Korean red ginseng), Panax notoginseng (Chinese ginseng), and Panax quinquefolius (American ginseng), have been used traditionally as aphrodisiacs and to promote sexual health. Preclinical and clinical trials have shown ginseng can increase sperm production and motility.152 In one clinical trial including 80 men with varicocele, some of whom were treated surgically, those who received 1,500 mg Panax ginseng powder daily for 12 weeks had greater improvements in sperm concentration, motility, morphology, and viability compared with placebo, and the combination of ginseng plus surgery led to the best effects.153 Another trial in 206 men with infertility related to chronic infectious prostatitis found those treated with ginseng plus L-carnitine and L-arginine for six months following 14 days of antibiotic therapy had higher sperm concentrations and increased sperm motility compared with those who received antibiotic therapy alone.154
Melatonin
Melatonin, a hormone produced in the brain that helps regulate the body’s circadian rhythms, plays a role in normal sperm production and protection against free radical damage, and low melatonin levels have been noted in men with infertility.155-157 Nighttime exposure to light, especially blue light, from electronic screens suppresses melatonin release and has been correlated with reduced sleep duration as well as lower sperm motility and concentration.158,159 Shift work, which impairs melatonin release and disrupts circadian cycles, has also been linked to poor sperm parameters and increased risk of infertility.160 Exposing sperm from men with infertility to a melatonin-enriched environment was found in one study to reduce DNA fragmentation and increase viability.161 In a placebo-controlled trial in 54 men who had undergone surgery for varicocele, men receiving melatonin had greater improvement in semen parameters, antioxidant capacity, and inhibin B levels compared with men receiving placebo after three months.162 In couples undergoing in vitro fertilization due to male infertility, giving the men 6 mg of melatonin daily was reported to reduce sperm DNA oxidative damage after 45 days and result in higher quality embryos.163
French Maritime Pine Bark
French maritime pine bark extract is rich in polyphenols with free radical-scavenging effects. In an uncontrolled trial, 19 men with infertility had improvement in sperm morphology and function after 90 days of treatment with pine bark extract at 200 mg per day.164 In a randomized crossover trial in 50 subjects with male infertility, one month of treatment with a supplement containing pine bark extract plus L-arginine (an amino acid precursor to nitric oxide which regulates blood vessel function) increased semen volume, sperm number and concentration, motility, and vitality, and improved sperm morphology compared with placebo.165 An uncontrolled trial that included 47 men with low sperm numbers and motility and mild erectile dysfunction reported a combination of 60 mg pine bark extract plus 690 mg L-arginine daily for four months improved sperm concentration as well as sexual function.166
Tribulus
Tribulus (Tribulus terrestris) is a plant that grows in parts of Asia, Europe, Australia, and Africa. Tribulus is high in compounds known as steroidal saponins, including protodioscin, which is thought to contribute to its therapeutic properties. Although popular reports of its ability to raise testosterone levels have led to its common use to treat problems related to male sexual function, including erectile dysfunction and low sexual desire, clinical trials have yielded mixed findings.167,168
A systematic review of seven clinical trials concluded tribulus can effectively improve sperm parameters.169 In one uncontrolled trial, 65 men with abnormal semen parameters received 250 mg tribulus (providing 37.5 mg protodioscin) three times daily. After 12 weeks, increased conversion of testosterone to the more potent androgen dihydrotestosterone, as well as improved sperm count and motility, were seen.170 However, in another uncontrolled trial, 30 men with infertility had no change in semen parameters, testosterone levels, or LH levels after three months of treatment with 250 mg tribulus extract three times daily.171
Trials in aging men with erectile dysfunction have found treatment with tribulus extract, at a dose of 250 mg three times daily for three months, can increase testosterone levels and improve erectile function without worsening lower urinary tract symptoms.172,173 However, 400 mg of tribulus extract twice daily for 30 days had no impact on testosterone levels or erectile function in one placebo-controlled trial.174 Research also suggests tribulus may not alter testosterone levels in men younger than 40 years old.175
Lipoic Acid
Alpha-lipoic acid, a compound found throughout the body and in many plant foods, has the capacity to regenerate antioxidants such as glutathione, vitamin C, and vitamin E.176 Some evidence suggests alpha-lipoic acid may enhance sperm quality and fertility.177
A controlled trial found all participants who had undergone surgery for varicocele had improvements in sperm parameters, but those given 600 mg alpha-lipoic acid per day for 80 days had greater improvement in sperm motility than those given placebo.178 Another controlled trial evaluated the effect of a supplement containing acetyl-L-carnitine, L-carnitine fumarate, and alpha-lipoic acid in 80 infertile men with increased sperm DNA fragmentation and oxidative stress. After three months the supplement group had decreased DNA fragmentation and oxidative stress and improved sperm motility and morphology.179
Spirulina
Spirulina (Spirulina platensis) is a blue-green microalgae best known for its strong free radical-quenching ability. Animal research suggests spirulina may counter the toxic effects of lead acetate on testicular function by reducing oxidative stress and inflammation.180 In one randomized controlled trial in 40 men with infertility, 2 grams of spirulina daily along with standard therapies for 12 weeks had no effect on semen parameters but resulted in a 5% higher pregnancy rate than placebo.181
Shilajit
Shilajit, a natural compound found in the Himalayas that is made from microbial decomposition of plants,182 has been used in traditional Ayurvedic medicine as an aphrodisiac and a treatment for male infertility.183 In an uncontrolled trial in 28 men with low sperm numbers, 100 mg shilajit extract daily for 90 days led to increased sperm numbers and motility. In addition, testosterone and FSH levels were higher and oxidative stress was lower at the end of the trial.184 In a mouse model of male infertility, shilajit improved testicular function, increased sperm and testosterone production, reversed the toxic effect of the heavy metal cadmium on sperm motility and concentration, and induced fertility.183
Saffron
Saffron (Crocus sativus) is a culinary and medicinal herb with carotenoid pigments that possess strong antioxidant capacities. Although findings from several randomized controlled trials indicate saffron may be beneficial in men with erectile dysfunction, there is mixed evidence for its potential benefit in male infertility.185 In an uncontrolled trial that included 52 men with infertility deemed not surgically treatable, 50 mg of saffron three times weekly for three months led to improvements in sperm motility and morphology, but not sperm counts.186 On the other hand, in a randomized controlled trial in 260 men with abnormal sperm number, motility, and morphology, 60 mg of saffron daily for 26 weeks resulted in no differences relative to placebo with regard to sperm density, motility, or morphology, or semen antioxidant capacity.187
Nutrient/Antioxidant Combinations
Treatment protocols implementing multi-nutrient combination formulas, particularly antioxidant combinations, have been found to improve male infertility in multiple clinical trials. A meta-analysis of findings from seven randomized controlled trials found evidence suggestive of a possible positive effect of antioxidant supplementation on clinical pregnancy and successful birth rates.188 A separate review suggests antioxidant supplementation can also improve semen quality and parameters such as concentration, motility, and morphology by attenuating oxidative stress-induced sperm damage.189 In an uncontrolled trial that included 119 men with idiopathic infertility, supplementation with a multi-nutrient cocktail containing vitamins, minerals, and several antioxidants twice daily for three months led to significant improvements in sperm concentration, motility, morphology, and DNA fragmentation compared with pretreatment analyses.190
Given the positive outcomes with L-carnitine and/or acetyl-L-carnitine, it has been included in combination with antioxidants and other nutrients. An open trial compared L-carnitine (500 mg twice daily) alone to 440 mg L-carnitine plus 250 mg L-arginine, 40 mg zinc, 120 mg vitamin E, 80 mg glutathione, 60 mcg selenium, 15 mg CoQ10, and 800 mcg folic acid per day. Both L-carnitine alone and in combination improved sperm parameters, but the combination was more effective for improving sperm concentration and motility.191 In one randomized controlled trial that included 175 men with infertility, taking a supplement providing 1 gram L-carnitine and 0.5 grams acetyl-L-carnitine, as well as 725 mg fumarate, 1 gram fructose, 50 mg citric acid, 10 mg zinc, 20 mg CoQ10, 50 mcg selenium, 90 mg vitamin C, 200 mcg folic acid, and 1.5 mcg B12, twice daily improved sperm motility, semen volume, and DNA fragmentation compared with placebo after six months.192 Another trial included 83 men with at least one abnormal sperm parameter who received either 2 grams of combined L-carnitine and acetyl-L-carnitine plus 250 mg L-arginine, 100 mg glutathione, 40 mg CoQ10, 7.5 mg zinc, 234 mcg folic acid, 2 mcg vitamin B12, and 50 mcg selenium or placebo daily for six months. After four months, sperm parameters normalized in 69% of those receiving the L-carnitine combination and 22% of those receiving placebo, and after six months, the spontaneous pregnancy rate was almost 24% in the supplement group versus almost 5% in the placebo group.193
Myo-inositol is another nutrient that has demonstrated positive effects alone and has been studied in combination with other nutrients. In an uncontrolled trial in 100 men with infertility, a combination supplement containing 800 mg alpha-lipoic acid, 100 mg betaine, 400 mcg folic acid, 1,000 mg myo-inositol, 2.6 mg B12, 1.7 mg riboflavin, and 1.9 mg B6 was tested and reported to improve sperm concentration, motility, and morphology.194 An uncontrolled trial that enrolled 109 men with low sperm motility found supplementing twice daily with 1 gram myo-inositol, 200 mcg folic acid, 55 mcg selenium, 30 mg L-arginine, 30 mg L-carnitine, and 30 mg vitamin E improved sperm motility in more than 85% of participants after three months.195 This same supplement not only improved sperm parameters but also was reported to increase testosterone levels and insulin sensitivity after three months in an uncontrolled trial in 45 men with low sperm motility and metabolic syndrome.196 Findings from a controlled trial support the effectiveness of this myo-inositol-based combination supplement, in conjunction with myo-inositol treatment of recovered sperm, for enhancing pregnancy likelihood in couples with male infertility undergoing intracytoplasmic sperm injection (ICSI) procedures.197
6 Diet & Lifestyle Changes to Support Male Fertility
Diet
There is increasing evidence that eating a healthy diet promotes normal fertility in both men and women. Markers of a poor diet, such as high intake of saturated fats and sugar, have been linked to lower male fertility, whereas markers of healthy eating, such as balanced intake of omega-3 and omega-6 fatty acids, high consumption of whole grains, and a diversity of fruits and vegetables providing ample amounts of essential vitamins and minerals, have been correlated with better sperm parameters.198 Scores showing strong adherence to the healthy dietary patterns including the Mediterranean, Prudent, and DASH (Dietary Approaches to Stop Hypertension) diets, all of which are characterized by high intakes of fruits, vegetables, and whole grains, have been associated with improved sperm parameters in studies involving men with infertility.199-201 On the other hand, dietary patterns associated with reduced male fertility are characterized by high intake of red and processed meats, sweet drinks and snacks, trans- and omega-6 fatty acids, and overall calories, as well as low intake of fiber, fish, fruits and vegetables, and nuts and seeds.60
Lifestyle
Lifestyle changes are an important aspect of male infertility treatment, particularly for men whose infertility has no known cause.
- Weight loss. Maintaining a healthy weight is one of the most important steps men can take to maintain or restore fertility. While obesity is linked to poor sperm quantity and quality and lower chance of successful pregnancy and birth, weight loss in obese men has been shown to rapidly improve sperm numbers, motility, morphology, and DNA fragmentation rate.36
- Smoking cessation. Infertile men who smoke may be able to increase their chances of having a child by quitting smoking.202 Smoking is associated with lower semen volume and total sperm count; however, cessation may have a restorative effect.203
- Reducing alcohol. Although moderate alcohol intake is not likely to impact fertility, heavy alcohol use is toxic to sperm and daily alcohol use has been linked to reduced male fertility.36,202 Limiting alcohol intake may therefore be beneficial to men wanting to improve fertility.
- Eliminating recreational drugs. Anabolic steroids, cannabis (marijuana), cocaine, methamphetamines, and opioids have all demonstrated negative impacts on male fertility, and their avoidance is essential to an effective fertility-enhancing program.204,205
- Sleep. Both too little (less than six hours per night) and too much (more than nine hours per night) sleep can negatively impact male fertility. Strategies to improve sleep (eg, targeting 7–8 hours per night) may improve male fertility markers.206
- Stress management. Psychological stress is extremely common in couples dealing with infertility. Unfortunately, stress can rapidly increase free radical production and oxidative stress, and chronic stress increases the risk of other physical and mental health problems.207,208 Therefore, efforts to reduce stress such as mindfulness and meditation practices may benefit men dealing with infertility.
Physical activity is part of a healthy lifestyle associated with fertility maintenance. Physically active men exhibit better semen parameters (semen volume and sperm viability, motility, and morphology) than sedentary counterparts.209 In a clinical trial in 90 obese adults, a 16-week aerobic training program consisting of a warmup (10‒15 minutes) and exercise (35‒50 minutes) using a treadmill three times per week improved sperm count, motility, and morphology, in addition to levels of serum testosterone.210
It is important to wear protective gear during physical activity as sports-related head injuries may contribute to hypogonadism (low testosterone levels), and sports like football, basketball, handball, and volleyball have been correlated with incidence and severity of varicocele.74 In addition, long periods of cycling increases testicular heat, inflammation, and oxidative stress.211
7 Diagnosing Male Infertility
Infertility evaluation is generally recommended for both male and female partners in couples who have been unable to conceive after 12 months of regular unprotected sexual intercourse.2 The diagnostic process begins with a thorough history. For the male partner, this would include1,2:
- Previous fertility and other reproductive issues
- Sexual activity, libido, and function
- Past and present medical conditions and medication usage
- Childhood conditions affecting the reproductive system, such as undescended testicle(s), mumps orchitis, and early puberty
- Physical or surgical trauma affecting the reproductive tract
- Alcohol, cigarette, and recreational drug use
- Occupation and other potential sources of environmental toxin exposure
- Family history of infertility or other medical conditions
- Physical examination
A screening physical exam can be helpful in identifying lesions, cysts, tumors, varicoceles, and structural abnormalities related to the penis, vas deferens, epididymis, and testicles that may require further assessment. Signs of hormone imbalance, such as decreased body or facial hair, underdeveloped testes, and breast enlargement, may also be noted during physical exam.1
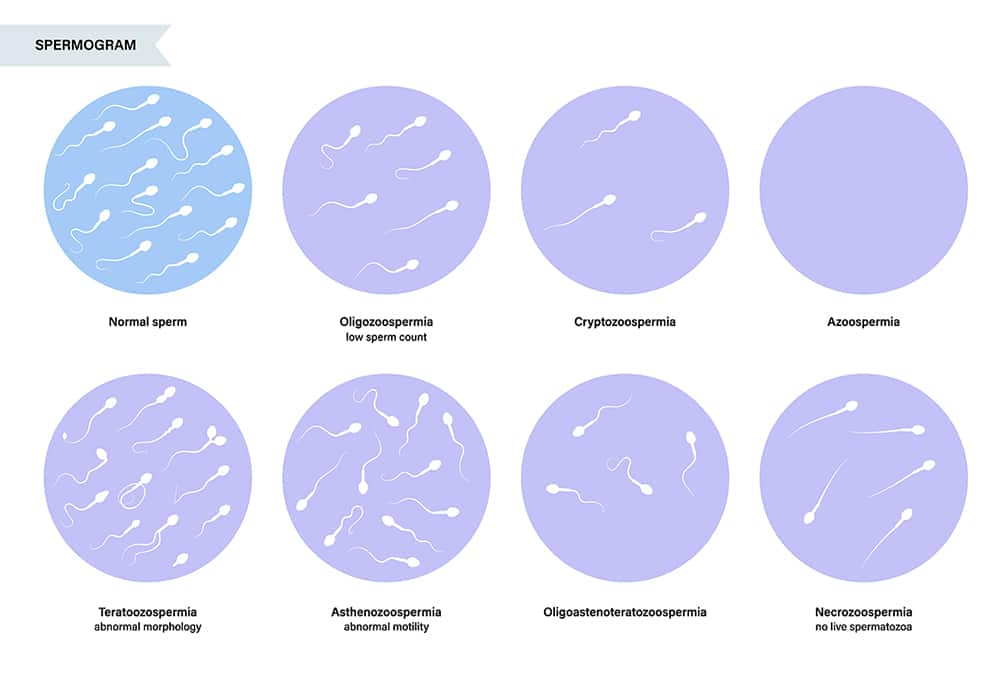
Semen Analysis
Semen analysis is an important screening test for diagnosing male infertility. The analysis is performed on ejaculate samples elicited on two occasions separated by at least one week and each following at least three days of abstinence.1 Samples are generally collected and analyzed in a lab, but home-based tests are also a reasonable option for preliminary screening and may be preferred for their enhanced privacy, convenience, and affordability.2,212 A semen analysis is done to assess semen volume, pH, and presence of white blood cells, immature germ cells, or other debris. The time needed for semen to liquefy from its initial gel-like state is evaluated as a reflection of enzyme activity. The number, concentration, vitality, motility, progression (forward movement, in either a straight line or large circles), and morphology of sperm are also assessed.1 Newer technologies used to evaluate DNA fragmentation and other sperm components, though not yet routinely used, may also inform the treatment approach.213
The immunobead test and sperm immobilization test are antisperm antibody tests performed on semen. It is particularly important to test for antisperm antibodies in men showing sperm agglutination (clumping), as well as those with normal sperm concentration but low sperm motility.214
Methodological differences between labs and variable results have hampered the usefulness of semen analysis. Computer assisted semen analysis systems, though expensive, have led to somewhat improved accuracy.212 Nevertheless, semen analysis does not identify all male infertility cases, since even in the context of normal findings, sperm may be unable to reach the female germ cell, fertilize it, or contribute to a healthy pregnancy.213 In fact, in 10–20% of cases of male infertility, semen parameters are normal and no other cause is found.1
Hormonal Profile
Hormone testing may be performed for any man with infertility, but is especially important in men with low sperm concentration, sexual dysfunction, or other signs of hormone imbalance.2 A comprehensive male infertility hormone profile includes1:
- Total and free testosterone
- Follicle stimulating hormone (FSH)
- Luteinizing hormone (LH)
- Estradiol
- Sex hormone binding globulin (SHBG)
- Prolactin
- Thyroid stimulating hormone (TSH)
Post-coital and Sperm Function Tests
Post-coital testing may be recommended in certain cases of unexplained infertility with normal sperm parameters. It involves examination of the female partner’s cervical mucous to identify the presence or absence of viable motile sperm. If viable sperm are seen, sperm function tests may be undertaken to more specifically identify abnormal sperm activity. Sperm function tests may be helpful in predicting the likelihood of success using assisted reproductive procedures.1
Post-ejaculatory Urinalysis
Checking urine for the presence of sperm may be done to confirm a diagnosis of retrograde ejaculation in men with low semen volume. Sperm collected during urinalysis can be used in assisted reproductive techniques.1
Genetic Screening
Genetic screening may be indicated for men with azoospermia or severe oligozoospermia. Relevant screening tests include karyotype analysis to identify abnormal chromosomal number or structure, cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation, and Y chromosome microdeletions.1,2 If genetic causes of infertility are identified, assisted reproductive techniques can be considered; however, some genetic defects reduce the odds of successful outcomes or can be passed on to children.1
Imaging
Ultrasound of the scrotum is sometimes indicated as part of male infertility evaluation, particularly for those cases with inconclusive physical exam findings. Scrotal ultrasound provides information about testicular size, volume, density, and blood flow, and can detect or confirm a testicular mass, absence of vas deferens, epididymal abnormalities, and varicocele.1,2 Scrotal ultrasound is also important in screening for testicular cancer in men with non-obstructive azoospermia.215 The risk of testicular cancer in men with infertility is about 0.5%, which, though low, is 100-fold higher than in the overall male population.1 There is also a 48% increased risk of prostate cancer in men diagnosed with infertility, although the absolute risk remains low.56
Transrectal ultrasound can be helpful in identifying an obstruction or other problems related to the seminal vesicles, prostate, or ejaculatory duct. Magnetic resonance imaging (MRI) is sometimes performed to obtain more detailed imaging of the reproductive tract.2
Vasography, an invasive technique involving injection of saline or a contrast material into the vas deferens, can be used to confirm patency (openness) or locate an obstruction and is generally performed prior to surgery.1,2
Testicular Biopsy
Testicular biopsy may be indicated in cases of azoospermia in which history, physical exam, semen analysis, laboratory tests, genetic screening, and imaging studies are inconclusive. Biopsy can help distinguish failure of sperm production from intratesticular obstruction and detect testicular cancer. Because testicular biopsy can damage the testes, its use is limited to highly specific cases and is often performed in conjunction with testicular sperm extraction for cryopreservation (freezing) and possible use in assisted reproductive strategies.215
8 Male Infertility Treatment
The treatment approach to male infertility is based on underlying cause. For example, appropriate antibiotics may be prescribed if infection is identified, and glucocorticoids may be recommended if antisperm antibodies are present.216 However, in as many as 70% of cases, there is no specific medical fertility treatment for abnormalities in sperm parameters. In these cases, empirical reproductive medicine (eg, drugs that may not have established records of effectiveness) or assisted reproductive techniques may be offered.
In addition to healthy diet and lifestyle changes described above, couples may benefit from counsel in sexual practices that increase the likelihood of pregnancy. These include ovulation tracking and engaging in sexual intercourse every 48 hours around the time of ovulation.2 Some vaginal lubricants appear to slow sperm motility or have sperm-toxic effects, and therefore should be avoided or used sparingly.217,218 Careful placement of electronic devices, such as placing a laptop on a table or desk instead of your lap, should be considered to avoid damage to sperm; avoidance of saunas, hot tubs, and tight underwear, all of which can reduce sperm quantity and quality, are also recommended.1,65
Hormonal Therapies
Hormone therapies are used to treat male infertility due to hormonal causes, such as hypogonadotropic hypogonadism and hyperprolactinemia.
- Testosterone. Testosterone therapy is contraindicated in men attempting to overcome infertility, even in those with low testosterone levels, due to its suppressive effect on the HPG axis. Unfortunately, it is frequently inappropriately prescribed.219,220 A possible exception is nasal testosterone gel, which has been found to have little effect on gonadotropin levels or semen parameters, and may be useful to improve sexual function in men with low testosterone levels.221,222
- Gonadotropins. Gonadotropins, including gonadotropin releasing hormone (GnRH), LH, FSH, and human chorionic gonadotropin (hCG) (a hormone with LH-like effects), are approved for use in treating infertility due to hypogonadotropic hypogonadism.219 GnRH is used in pulses administered via a pump device implanted under the skin to induce fertility in men with normal pituitary function. Combinations of gonadotropins have been found to be equally effective for treating hypogonadotropic hypogonadism, and are less expensive and less invasive than pulsatile GnRH therapy.17 In particular, the use of hCG plus FSH for one to two years has been found to improve sperm production in about 80% of men and result in a pregnancy rate of approximately 50%.223 Gonadotropin therapies are expensive and sometimes cause breast enlargement.17,219
- Selective estrogen receptor modulators. Clomiphene citrate is a selective estrogen receptor modulator (SERM) that increases gonadotropin release by decreasing estrogen signaling. The increase in gonadotropins stimulates testicular activity, including sperm production. Clomiphene citrate is widely used to treat male infertility due to hypogonadotropic hypogonadism, and has been shown to increase successful pregnancy rates.219,224 It is sometimes prescribed along with tamoxifen, another SERM, which may enhance its effectiveness.1 The most common side effects of SERM therapies are digestive upset, dizziness, hair loss, breast enlargement, and weight gain.219
- Aromatase inhibitors. Aromatase inhibitors block the conversion of testosterone to estrogen and are used experimentally to treat hormone-related male infertility. Letrozole (Femara) and anastrozole (Arimidex) are examples of aromatase inhibitors sometimes used to treat infertility in men with low testosterone levels. Although their effectiveness for increasing pregnancy rates is not known, they appear to improve hormone levels and sperm parameters.1 Aromatase inhibitors are thought to be most helpful in men with a low testosterone and relatively high estrogen levels, and are sometimes used in conjunction with clomiphene citrate.1,219 Aromatase inhibitors are generally well tolerated but can trigger side effects including decreased libido, headache, hot flashes, weight gain, and insomnia, as well as liver enzyme elevation and bone loss.17,219
- Dopamine agonists. Dopamine agonists, including bromocriptine (Cycloset), lisuride (Dopergin), quinagolide (Norprolac), and cabergoline (Dostinex), are used to treat hyperprolactinemia and are often needed long term.225 These medications can cause side effects such as headaches, low blood pressure, and nausea.17 Alternatively, surgery to remove the prolactin-secreting tumor (a non-cancerous tumor in the pituitary gland) has also been shown to successfully lead to remission.226
Other Medical Treatments
Drugs that help strengthen the bladder neck muscle by activating the sympathetic nervous system may be helpful in treating some cases of retrograde ejaculation and anejaculation.1 These include imipramine (Tofranil), midodrine (ProAmatine), chlorpheniramine (Chlor-Trimeton), brompheniramine (Veltane), ephedrine (Akovaz), pseudoephedrine (Sudafed), and phenylephrine (Vazculep). These medications can cause side effects related to sympathetic nervous stimulation, such as loss of appetite, digestive upset, tremor, insomnia, and arrhythmia.227 However, in some cases of retrograde ejaculation, surgery or sperm retrieval for assisted reproductive techniques are the best option.228
Glucocorticoids such as prednisolone (Prednisone) are sometimes used to treat men with infertility expressing antisperm antibodies. This treatment has been reported to improve sperm parameters, pregnancy rates, and in vitro fertilization outcomes.229-231
Medical and other treatments are also available for men with infertility related to erectile dysfunction. For more details, please see the Erectile Dysfunction protocol.
Idiopathic Infertility: Empirical Medical Therapies
Empirical medical therapies (drugs that do not necessarily have established records of effectiveness) are often used to treat idiopathic male infertility.2 These include:
- Gonadotropins. While the use of gonadotropins is approved for treatment of hypogonadotropic hypogonadism, their possible benefit in men with infertility related to idiopathic sperm abnormalities is still under investigation.220 Although findings from clinical trials have been mixed, some evidence suggests FSH therapy, particularly using high doses, may enhance sperm quantity and quality and improve pregnancy rates in men with idiopathic infertility.232,233 A single controlled trial in 19 men with idiopathic infertility found hCG, used in conjunction with human menopausal gonadotropin (a combination of LH and FSH), for three months resulted in a higher pregnancy rate than placebo, and positive effects on sperm parameters were found to be correlated with higher inhibin B levels.234,235 These findings need to be confirmed in larger trials.
- SERMs. Because clomiphene citrate and tamoxifen increase testicular function by increasing gonadotropin signaling, these drugs are sometimes used empirically to treat idiopathic male infertility.1 A meta-analysis of findings from 11 randomized controlled trials found SERM therapy with either clomiphene citrate or tamoxifen improved some sperm parameters and hormone levels, and more than doubled the pregnancy rate in couples affected by idiopathic male infertility.236 Another meta-analysis that included 16 trials similarly noted improved sperm numbers, concentration, hormone levels, and pregnancy rates in men with idiopathic infertility treated with SERMs.237
- Aromatase inhibitors. By blocking estrogen production, aromatase inhibitors reduce negative feedback signaling and thereby increase gonadotropin release by the pituitary gland. In an open uncontrolled trial in 20 men with idiopathic infertility and a low testosterone-to-estrogen ratio, three months of treatment with letrozole resulted in improved sperm motility and morphology, reduced sperm DNA fragmentation, increased testosterone-to-estrogen ratio, and four spontaneous pregnancies.238 Another uncontrolled study found 15 idiopathic male infertility patients with low sperm concentrations and normal testosterone-to-estrogen ratio had increased sperm concentrations after four months of treatment with letrozole.239 The possible benefits of letrozole therapy need more research.
Surgical Approaches
Various surgical procedures can be beneficial in cases of varicocele and obstructive causes of male infertility. Sperm retrieval can be performed with each of these surgeries, allowing for the option of assisted reproductive techniques should surgery be unsuccessful in restoring fertility.
- Varicocelectomy. Varicocele repair (varicocelectomy) is indicated in infertile men with large varicoceles that are readily apparent on physical exam, and improves semen parameters in 60–70% of these patients. Some evidence suggests varicocelectomy may still be beneficial in select patients with smaller varicoceles; in particular, those with smaller testicular volume and decreased testicular blood flow. Men with evidence of other co-existing causes of infertility are less likely to benefit from this surgery.1 A procedure known as microscopic subinguinal varicocelectomy has been shown to have the highest success rate and lowest risk of complications. This surgery may be facilitated by Doppler ultrasound, angiography, or video microscopy to visualize the vasculature.240 According to one meta-analysis, microscopic subinguinal varicocelectomy resulted in a natural (unassisted) pregnancy rate of 27.5% in men with severe oligozoospermia prior to surgery.241
- Transurethral Resection of the Ejaculatory Duct (TURED). This surgery is indicated for men with ejaculatory duct obstructions seen on transrectal ultrasound. Possible complications include urinary incontinence and epididymitis. In some cases, vesiculoscopy, a procedure in which the ejaculatory duct is temporarily dilated, may allow for natural conception without surgery.240
- Vasovasostomy and Vasoepididymostomy. These microsurgical procedures are indicated to repair bilateral obstruction in the vas deferens or epididymis, such as due to vasectomy (contraceptive surgery in which the vas deferens are severed). Inflammation and scarring may limit the success of these procedures.1 Advanced technologies involving robotic assistance have been reported to increase pregnancy rates following vasovasostomy from 55% to 65%, but add expense.242
Assisted Reproductive Techniques
Assisted reproductive techniques are recommended to couples with untreatable male infertility, such as due to genetic mutations or idiopathic causes, and those for whom indicated treatments are unsuccessful. Sperm retrieval is an essential part of all assisted reproductive techniques. Semen can be a source of sperm if some healthy, motile sperm are present; however, in cases of azoospermia, severe oligozoospermia, or severely abnormal sperm parameters, sperm may be obtained via aspiration of the epididymis, vas deferens, or testes, or through microsurgical dissection of the testes.243 Sperm retrieved through testicular microdissection are generally housed in the seminiferous tubules where they were produced and are not yet motile; therefore, they require extensive manipulation and processing before individual sperm can be selected and used in assisted reproductive techniques.244 Novel methods for selecting the most viable and healthy sperm are under investigation and may improve outcomes in couples with severe sperm abnormalities in the future243,245,246; however, they have not yet been shown to enhance fertilization, implantation, or successful birth rates, and add to the cost and invasiveness of assisted reproductive techniques.247
When considering whether or not to pursue assisted reproductive techniques, it is worth bearing in mind that these procedures come with known and theoretical risks. Known risks include multiple pregnancies (eg, twins or triplets) and related pregnancy complications, as well as side effects in the female partner when ovulation-inducing drugs are used.248 Theoretical risks include health problems in offspring related to genetic and epigenetic effects of oxidative stress, which may occur as a result of the procedure or may be inherited via the infertile father.249 Observational studies have found a higher incidence of birth defects in babies conceived via assisted reproductive technique, especially those conceived using intracytoplasmic sperm injection (ICSI).250-252 In addition, assisted reproductive technique-conceived babies have been found to be more likely to experience perinatal problems (eg, low birth weight and preterm birth) compared with those conceived naturally, and some evidence suggests they may be at higher risk of cardiovascular problems later in life.252 A retrospective cohort study of 112,043 pregnant women and their 114,522 newborns in China found that compared to spontaneously conceived infants, those conceived with assisted reproductive techniques had more than twice the risk of having any birth defect.253 Although more than a third of these defects were related to multiple pregnancies, the remainder were considered to be direct effects of assisted reproductive techniques.
Another important consideration is the psychological toll to both partners of pursuing expensive, invasive procedures without a guarantee of success. Stress, anxiety, and depression are common in men and women being treated for infertility, and psychological support has been shown to improve couples’ mental health and well-being.254 While some research suggests psychosocial interventions may increase pregnancy rates in infertile couples, it is perhaps more important that psychosocial support improves acceptance of outcomes.254,255 This may help couples to adapt to a child-free life or begin exploring the possibility of adoption.
Intrauterine insemination. Intrauterine insemination involves the separation of motile sperm from semen and its placement directly in the uterus around the time of ovulation.1 The use of ovulation-inducing medications by the female partner prior to intrauterine insemination appears to increase the likelihood of pregnancy but also increases the risk of pregnancy with twins, triplets, or other multiples, as well as a serious complication called ovarian hyperstimulation syndrome.256 Most studies suggest pregnancy is unlikely if the total motile sperm count is under 5 million, and some research indicates those with total motile sperm counts of 10–30 million may have the best chance of successful intrauterine insemination.257 In addition, the chance of pregnancy is lower if damage to motility or morphology affects a large percentage of sperm, abnormalities in sperm function are identified, or the female partner is over 40 years old. In general, the successful pregnancy rate with intrauterine insemination is about 12% per attempt but decreases with each successive attempt. Although the pregnancy rate is lower than with in vitro fertilization and intracytoplasmic sperm injection, intrauterine insemination is often recommended first (when feasible) due to its lower invasiveness and cost.1
In vitro fertilization. In vitro fertilization (IVF) involves the fertilization of a mature oocyte (egg) outside of the female body. IVF is available to couples facing male infertility for whom intrauterine insemination is not feasible or unsuccessful. The harvesting of eggs for IVF is usually facilitated by ovulation-inducing medications. Typically, about 12 eggs are harvested and placed in a fertilization medium with 50,000 to 500,000 motile sperm. If the number of motile sperm is too low, intracytoplasmic sperm injection can be used. After two days, 2–4 embryos (successfully fertilized and growing oocytes) are implanted in the female partner and the rest are frozen.1 IVF has been reported to be associated with a pregnancy rate of 3.1–47.6%, depending on the age of the female partner. Because IVF involves the use of ovulation-inducing medications, it carries an increased risk of ovarian hyperstimulation syndrome, a potentially life-threatening complication. IVF also increases the likelihood of multiple pregnancies, which may pose maternal pregnancy-related hazards and are linked to increased risk of preterm birth.248
Intracytoplasmic sperm injection. Intracytoplasmic sperm injection (ICSI), a form of IVF in which a single mature sperm is injected into the cytoplasm of a mature oocyte in the laboratory, is the most widely used assisted reproductive technique in the world. The use of intracytoplasmic sperm injection has led to better IVF outcomes in couples with male infertility, though its use in cases without a male infertility factor are still controversial.258,259 Intracytoplasmic sperm injection results in successful pregnancy and birth in about 30% of cases overall, and evidence suggests sperm DNA fragmentation may play a role in limiting positive outcomes. Sperm retrieved from the testes have been found to have less DNA fragmentation than sperm from ejaculate and may result in better intracytoplasmic sperm injection outcomes for men with high levels of sperm DNA fragmentation.260
9 Living with Male Infertility
Dealing with male infertility can be a long and emotionally demanding journey. First, one must experience lack of conception for a full year before being diagnosed as infertile; then, investigations into possible causes can take time and may feel invasive and defeating. Lengthy treatment protocols, an inability to afford expensive interventions (especially assisted reproductive techniques), and financial difficulties incurred through treatment can add to the burden of stress.62,261 Furthermore, unfortunately, only about 50% of infertile couples become pregnant after treatment.62 Possibly for these and other reasons, not pursuing medical treatment for infertility has been correlated with less emotional distress.262
Infertility is a serious cause of psychological and emotional distress, often prompting reactions such as shock, a sense of losing control, low self-esteem, anger, frustration, sadness, and grief, and the emotional toll increases the longer infertility persists.62,263 Infertility can also cause stress in relationships with partners, family members, and friends.62 Seeking support while coping with infertility can help individuals and couples find relief and regain their quality of life.255,264 In one study, participation in an online forum helped people dealing with infertility to feel less loneliness and stress.265 Couples who choose not to engage in treatment or are unable to conceive with treatment may benefit from ongoing support that allows them to fully process disappointment and grief. This may help them find acceptance of not having children or begin to consider the possibility of adoption.266,267
After fully processing the grief of infertility and coming to a point of acceptance, some couples will find adoption is a fulfilling way to become parents and grow a family. If you are considering adoption, here are some resources that may be helpful:
10 Frequently Asked Questions About Male Infertility
What is male infertility?
Male infertility is a disorder of the male reproductive system that results in an inability to bring about pregnancy. Infertility is medically defined as the inability of a couple to become pregnant after one year of regular sexual intercourse without the use of contraception. Issues with the male partner’s fertility are estimated to contribute to up to half of infertility cases.2
What is the main cause of male infertility?
The majority of infertile men have low sperm counts, poor sperm movement, and/or abnormally shaped sperm. Although there are many genetic and environmental factors that can lower sperm numbers or damage sperm, a specific cause of male infertility is often not identified.
Can male infertility be treated?
Sometimes. In some cases, the cause of male infertility can be treated with medical, surgical, or integrative treatments. An accurate assessment of the couple looking to conceive is necessary to guide decision-making around treatment options. Talk to your healthcare provider to determine whether treatment is appropriate.
How can a man tell if he is fertile?
A semen analysis is the first step in determining if a man is fertile. Importantly, both partners in a couple having trouble conceiving should have their fertility evaluated.
Can a man go from infertile to fertile?
Yes, some men with infertility become fertile. In some cases, this happens spontaneously, but more often it happens as a result of supportive dietary and lifestyle changes, nutritional therapies, medication, or surgery.
What health problems can contribute to male infertility?
Many chronic conditions can contribute to male infertility by increasing inflammation and free radical levels. Obesity, diabetes, cystic fibrosis, thyroid disease, and cancer are examples of health problems known to increase the risk of male infertility.
Can cigarette smoke affect sperm?
Yes. Smoking reduces the number of sperm, their ability to move, and their likelihood of having normal shape and structure. Smoking also causes DNA damage and genetic mutations in sperm, making them less able to successfully fertilize eggs. Electronic cigarette use may also lower male fertility, but this needs more research.
Can marijuana cause male infertility?
Possibly. Based on a growing body of evidence, it appears marijuana use may have a negative impact on male fertility by altering hormone signaling and interfering with sperm production and function. However, the extent to which marijuana plays a role in male infertility remains unclear.
Can using steroids for body building cause infertility?
Yes. Anabolic steroids (exogenous [from outside the body] testosterone and testosterone-like drugs) used by some athletes and body builders suppress hormonal signals from the brain that are needed to stimulate normal sperm production and maturation.
Do abnormal semen analyses or sperm lead to children with birth defects?
Maybe. Children born via assisted reproductive techniques, especially in vitro fertilization with intracytoplasmic sperm injection, have been noted to have higher rates of birth defects than children born via natural conception, but it is still not known whether this reflects problems caused by abnormal sperm, laboratory processing, or other factors.
What are assisted reproductive techniques?
Assisted reproductive techniques are procedures used to increase the odds of fertilization by introducing sperm directly into the uterus (intrauterine insemination), exposing eggs to sperm in a fertilization medium in the laboratory (in vitro fertilization, IVF), or injecting a single sperm directly into an egg (intracytoplasmic sperm injection).
Are there risks with IVF/intracytoplasmic sperm injection?
Yes. IVF and intracytoplasmic sperm injection (ICSI) poses the risk of testicular damage in men during sperm retrieval and medication side effects in women during egg harvesting. It also increases the odds of having twins, triplets, or other multiple pregnancies, which are associated with more pregnancy complications, preterm births, and birth defects.
Can environmental pollutants cause male infertility?
Yes. Pollutants known broadly as endocrine disruptors, including bisphenols, phthalates, organophosphates, mercury, and chemicals in natural gas, have been shown to reduce male fertility. Pollutants like radiofrequency electromagnetic radiation and air pollution may contribute to male infertility by causing sperm DNA damage.
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
References
- Leslie SW, Siref LE, Soon-Sutton TL, Khan MAB. Male Infertility. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Agarwal A, Baskaran S, Parekh N, et al. Male infertility. Lancet. Jan 23 2021;397(10271):319-333. doi:10.1016/S0140-6736(20)32667-2. https://www.ncbi.nlm.nih.gov/pubmed/33308486
- Walker MH, Tobler KJ. Female Infertility. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Agarwal A, Majzoub A, Parekh N, Henkel R. A Schematic Overview of the Current Status of Male Infertility Practice. World J Mens Health. Jul 2020;38(3):308-322. doi:10.5534/wjmh.190068. https://www.ncbi.nlm.nih.gov/pubmed/31385475 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7308239/pdf/wjmh-38-308.pdf
- Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv Nutr. Nov 1 2018;9(6):833-848. doi:10.1093/advances/nmy057. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6247182/pdf/nmy057.pdf
- Salas-Huetos A, James ER, Aston KI, Jenkins TG, Carrell DT. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod Biol. Sep 2019;19(3):219-224. doi:10.1016/j.repbio.2019.07.005. https://www.ncbi.nlm.nih.gov/pubmed/31375368
- Gurung P, Yetiskul E, Jialal I. Physiology, Male Reproductive System. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2022.
- JHM. Johns Hopkins Medicine. Overview of the Male Anatomy. Available at https://www.hopkinsmedicine.org/health/wellness-and-prevention/overview-of-the-male-anatomy. Accessed 09/20/21. 2021;
- Ivell R. Lifestyle impact and the biology of the human scrotum. Reproductive biology and endocrinology : RB&E. Apr 20 2007;5:15. doi:10.1186/1477-7827-5-15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1863418/pdf/1477-7827-5-15.pdf
- Jiann BP. The office management of ejaculatory disorders. Transl Androl Urol. Aug 2016;5(4):526-40. doi:10.21037/tau.2016.05.07. https://www.ncbi.nlm.nih.gov/pubmed/27652225 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5001990/pdf/tau-05-04-526.pdf
- DHHS. U.S. Department of Health and Human Services. Understanding Fertility: The Basics. Office of Population Affairs. Accessed 2/10/2022, https://opa.hhs.gov/reproductive-health/understanding-fertility-basics
- Herati AS, Kohn TP, Kassiri B. New frontiers in fertility preservation: a hypothesis on fertility optimization in men with hypergonadotrophic hypogonadism. Transl Androl Urol. Mar 2020;9(Suppl 2):S171-S177. doi:10.21037/tau.2019.12.39. https://www.ncbi.nlm.nih.gov/pubmed/32257857 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108987/pdf/tau-09-S2-S171.pdf
- Cohen J, Nassau DE, Patel P, Ramasamy R. Low Testosterone in Adolescents & Young Adults. Frontiers in endocrinology. 2019;10:916. doi:10.3389/fendo.2019.00916. https://www.ncbi.nlm.nih.gov/pubmed/32063884 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6966696/pdf/fendo-10-00916.pdf
- Millar AC, Faghfoury H, Bieniek JM. Genetics of hypogonadotropic hypogonadism. Transl Androl Urol. Mar 2021;10(3):1401-1409. doi:10.21037/tau.2020.03.33. https://www.ncbi.nlm.nih.gov/pubmed/33850776
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8039576/pdf/tau-10-03-1401.pdf - Sharma A, Mollier J, Brocklesby RWK, Caves C, Jayasena CN, Minhas S. Endocrine-disrupting chemicals and male reproductive health. Reprod Med Biol. Jul 2020;19(3):243-253. doi:10.1002/rmb2.12326. https://www.ncbi.nlm.nih.gov/pubmed/32684823 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7360961/pdf/RMB2-19-243.pdf
- Krzastek SC, Farhi J, Gray M, Smith RP. Impact of environmental toxin exposure on male fertility potential. Transl Androl Urol. Dec 2020;9(6):2797-2813. doi:10.21037/tau-20-685. https://www.ncbi.nlm.nih.gov/pubmed/33457251 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7807371/pdf/tau-09-06-2797.pdf
- Ide V, Vanderschueren D, Antonio L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. International journal of molecular sciences. Dec 22 2020;22(1)doi:10.3390/ijms22010021. https://www.ncbi.nlm.nih.gov/pubmed/33375030 https://mdpi-res.com/d_attachment/ijms/ijms-22-00021/article_deploy/ijms-22-00021.pdf
- Puzuka A, Alksere B, Gailite L, Erenpreiss J. Idiopathic Infertility as a Feature of Genome Instability. Life (Basel). Jun 29 2021;11(7)doi:10.3390/life11070628. https://www.ncbi.nlm.nih.gov/pubmed/34209597 https://mdpi-res.com/d_attachment/life/life-11-00628/article_deploy/life-11-00628.pdf
- Kuroda S, Usui K, Sanjo H, et al. Genetic disorders and male infertility. Reprod Med Biol. Oct 2020;19(4):314-322. doi:10.1002/rmb2.12336. https://www.ncbi.nlm.nih.gov/pubmed/33071633
- Adegoke EO, Rahman MS, Pang MG. Bisphenols Threaten Male Reproductive Health via Testicular Cells. Frontiers in endocrinology. 2020;11:624. doi:10.3389/fendo.2020.00624. https://www.ncbi.nlm.nih.gov/pubmed/33042007 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7518410/pdf/fendo-11-00624.pdf
- Pivonello C, Muscogiuri G, Nardone A, et al. Bisphenol A: an emerging threat to female fertility. Reproductive biology and endocrinology : RB&E. Mar 14 2020;18(1):22. doi:10.1186/s12958-019-0558-8. https://www.ncbi.nlm.nih.gov/pubmed/32171313 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7071611/pdf/12958_2019_Article_558.pdf
- Fang Y, Su Y, Xu J, et al. Varicocele-Mediated Male Infertility: From the Perspective of Testicular Immunity and Inflammation. Front Immunol. 2021;12:729539. doi:10.3389/fimmu.2021.729539. https://www.ncbi.nlm.nih.gov/pubmed/34531872 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8438154/pdf/fimmu-12-729539.pdf
- Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Research. 2019;8doi:10.12688/f1000research.17076.1. https://www.ncbi.nlm.nih.gov/pubmed/31143441 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6524745/pdf/f1000research-8-18670.pdf
- Leslie SW, Sajjad H, Villanueva CA. Cryptorchidism. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Leslie SW, Sajjad H, Villanueva CA. Cryptorchidism. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the Factors Involved in Male Infertility: A Prospective Review. International journal of general medicine. 2020;13:29-41. doi:10.2147/IJGM.S241099. https://www.ncbi.nlm.nih.gov/pubmed/32104049 https://www.dovepress.com/getfile.php?fileID=55915
- Fijak M, Pilatz A, Hedger MP, et al. Infectious, inflammatory and 'autoimmune' male factor infertility: how do rodent models inform clinical practice? Human reproduction update. Jul 1 2018;24(4):416-441. doi:10.1093/humupd/dmy009. https://www.ncbi.nlm.nih.gov/pubmed/29648649 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6016649/pdf/dmy009.pdf
- Choe SA, Kim S, Im C, et al. Nighttime environmental noise and semen quality: A single fertility center cohort study. PLoS One. 2020;15(11):e0240689. doi:10.1371/journal.pone.0240689.
- Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertility and sterility. Nov 2015;104(5):1051-60. doi:10.1016/j.fertnstert.2015.08.033. https://www.ncbi.nlm.nih.gov/pubmed/26385403 https://escholarship.org/content/qt8pj0c0b9/qt8pj0c0b9.pdf?t=o0h7ly
- Gray M, Zillioux J, Khourdaji I, Smith RP. Contemporary management of ejaculatory dysfunction. Transl Androl Urol. Aug 2018;7(4):686-702. doi:10.21037/tau.2018.06.20. https://www.ncbi.nlm.nih.gov/pubmed/30211060 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6127532/pdf/tau-07-04-686.pdf
- Capogrosso P, Jensen CFS, Rastrelli G, et al. Male Sexual Dysfunctions in the Infertile Couple-Recommendations From the European Society of Sexual Medicine (ESSM). Sex Med. Jun 2021;9(3):100377. doi:10.1016/j.esxm.2021.100377. https://www.ncbi.nlm.nih.gov/pubmed/34090242 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8240351/pdf/main.pdf
- Wigby S, Suarez SS, Lazzaro BP, Pizzari T, Wolfner MF. Sperm success and immunity. Curr Top Dev Biol. 2019;135:287-313. doi:10.1016/bs.ctdb.2019.04.002. https://www.ncbi.nlm.nih.gov/pubmed/31155361 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6784542/pdf/nihms-1053363.pdf
- Nguyen-Powanda P, Robaire B. Oxidative Stress and Reproductive Function in the Aging Male. Biology. Sep 11 2020;9(9)doi:10.3390/biology9090282. https://www.ncbi.nlm.nih.gov/pubmed/32932761 https://mdpi-res.com/d_attachment/biology/biology-09-00282/article_deploy/biology-09-00282.pdf
- Eisenberg ML, Meldrum D. Effects of age on fertility and sexual function. Fertility and sterility. Feb 2017;107(2):301-304. doi:10.1016/j.fertnstert.2016.12.018. https://www.ncbi.nlm.nih.gov/pubmed/28160919 https://www.fertstert.org/article/S0015-0282(16)63090-X/pdf
- Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS. Smoking and Male Infertility: An Evidence-Based Review. The world journal of men's health. 2015;33(3):143-160. doi:10.5534/wjmh.2015.33.3.143. https://pubmed.ncbi.nlm.nih.gov/26770934 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4709430/
- Balawender K, Orkisz S. The impact of selected modifiable lifestyle factors on male fertility in the modern world. Cent European J Urol. 2020;73(4):563-568. doi:10.5173/ceju.2020.1975. https://www.ncbi.nlm.nih.gov/pubmed/33552585 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7848840/pdf/CEJU-73-1975.pdf
- Evans EPP, Scholten JTM, Mzyk A, et al. Male subfertility and oxidative stress. Redox biology. Oct 2021;46:102071. doi:10.1016/j.redox.2021.102071. https://www.ncbi.nlm.nih.gov/pubmed/34340027 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8342954/pdf/main.pdf
- Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. International journal of molecular sciences. Sep 17 2021;22(18)doi:10.3390/ijms221810043. https://www.ncbi.nlm.nih.gov/pubmed/34576205 https://mdpi-res.com/d_attachment/ijms/ijms-22-10043/article_deploy/ijms-22-10043-v2.pdf
- Asadi A, Ghahremani R, Abdolmaleki A, Rajaei F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int J Reprod Biomed. Jun 2021;19(6):493-504. doi:10.18502/ijrm.v19i6.9371. https://www.ncbi.nlm.nih.gov/pubmed/34401644 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8350854/pdf/ijrb-19-493.pdf
- Torres-Arce E, Vizmanos B, Babio N, Márquez-Sandoval F, Salas-Huetos A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology. Mar 20 2021;10(3)doi:10.3390/biology10030241. https://mdpi-res.com/d_attachment/biology/biology-10-00241/article_deploy/biology-10-00241-v2.pdf
- Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian journal of urology : IJU : journal of the Urological Society of India. Jul-Sep 2017;33(3):207-214. doi:10.4103/iju.IJU_15_17. https://www.ncbi.nlm.nih.gov/pubmed/28717270 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5508431/pdf/IJU-33-207.pdf
- De Luca MN, Colone M, Gambioli R, Stringaro A, Unfer V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants (Basel, Switzerland). Aug 13 2021;10(8)doi:10.3390/antiox10081283. https://www.ncbi.nlm.nih.gov/pubmed/34439531 https://mdpi-res.com/d_attachment/antioxidants/antioxidants-10-01283/article_deploy/antioxidants-10-01283-v2.pdf
- Leisegang K, Sengupta P, Agarwal A, Henkel R. Obesity and male infertility: Mechanisms and management. Andrologia. Feb 2021;53(1):e13617. doi:10.1111/and.13617. https://www.ncbi.nlm.nih.gov/pubmed/32399992 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/and.13617?download=true
- Barkabi-Zanjani S, Ghorbanzadeh V, Aslani M, Ghalibafsabbaghi A, Chodari L. Diabetes mellitus and the impairment of male reproductive function: Possible signaling pathways. Diabetes Metab Syndr. Sep - Oct 2020;14(5):1307-1314. doi:10.1016/j.dsx.2020.07.031. https://www.ncbi.nlm.nih.gov/pubmed/32755827
- Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Human reproduction update. Jan 1 2018;24(1):86-105. doi:10.1093/humupd/dmx033. https://www.ncbi.nlm.nih.gov/pubmed/29136166
https://watermark.silverchair.com/dmx033.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAsYwggLCBgkqhkiG9w0BBwagggKzMIICrwIBADCCAqgGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMolVznEpwxLrRXm5zAgEQgIICeXUEIUl6WQchESkY7MNNdN_NffvcfiGCGKEn51pTpdGqczEQ7Cuwx8Cd0zNW1jss19usWQWeX1IZDq5aHtXWoS0RI23z18ETy0ZB9OikqtNqwJxxWEaE30T3XGol-PWD3lMDhi9-okn5TlclWKIwCgiY-Hp5_F2h4psoxLIbO7Vpd03-a2K4CqOmxvMzq87pankp-uXu8ECQyU8a3PlAw-X2E7Ix2SoKzIbMmxKBYecW9kTsXFJVCrHu1Thid6WafwWLR4nQFwznim05f-jM_iC8H-0QXbPfhElknHHKhls_wB4m-a2p2iJ8L09pw5xr45Izl0YDfNHGExCx03aEWATO-P2wSFCSsVr6AhVN3aHz9QK1efZaIcXUZL2W0F5BFnVYmcQoM5w0Wu6zuTOJdHFf30ULkyKXlQuIGHXy_iKxhU-j4VW6NmemngN48lR7dKooVAsBew6DqmjuEKToYptKYVVYViQonBN4bJLBSh94GqVkZ52Jm3uzdTU8oere-bg27m2Of8VstB9Ikm3dLs8QdDpLzaAkfoGTb1AoGWZ7I2UjN-yWB0EVTCO5r885Ljad0lNSY4HarA0HV0aczVw3WDV49sa43B6l932Yhb0xCBjzU-TbPxhvS7f-FI8cXemPDw84izaQ2cZ2G3c2Hi5l6Drc_iKvXCFn_4_t_QxOqpSz5Lztlf0eIWeAhCJFo1a52CQPowD0vOKXUnqwEwXedqGO26Cxl-h0VYLnyd7sXOGvabezao73LwbXvD4crE6cFVCuqZPhn9Gcv1szDWZoUMWFRvYPNrMc5m006SZ5cZaMFs0OLulZDtdZhgLCdJFowRbeZfcpYw - Abbasihormozi SH, Babapour V, Kouhkan A, et al. Stress Hormone and Oxidative Stress Biomarkers Link Obesity and Diabetes with Reduced Fertility Potential. Cell J. Oct 2019;21(3):307-313. doi:10.22074/cellj.2019.6339. https://www.ncbi.nlm.nih.gov/pubmed/31210437 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6582426/pdf/Cell-J-21-307.pdf
- Zhu JL, Cai YQ, Long SL, Chen Z, Mo ZC. The role of advanced glycation end products in human infertility. Life Sci. Aug 15 2020;255:117830. doi:10.1016/j.lfs.2020.117830. https://www.ncbi.nlm.nih.gov/pubmed/32450172
- Omolaoye TS, du Plessis SS. Male infertility: A proximate look at the advanced glycation end products. Reprod Toxicol. Apr 2020;93:169-177. doi:10.1016/j.reprotox.2020.02.002. https://www.ncbi.nlm.nih.gov/pubmed/32050094
- La Vignera S, Vita R. Thyroid dysfunction and semen quality. International journal of immunopathology and pharmacology. Jan-Dec 2018;32:2058738418775241. doi:10.1177/2058738418775241. https://www.ncbi.nlm.nih.gov/pubmed/29737216 https://journals.sagepub.com/doi/pdf/10.1177/2058738418775241
- Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet. Jun 5 2021;397(10290):2195-2211. doi:10.1016/S0140-6736(20)32542-3. https://www.ncbi.nlm.nih.gov/pubmed/34090606 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32542-3/fulltext
- Bieniek JM, Lapin CD, Jarvi KA. Genetics of CFTR and male infertility. Transl Androl Urol. Mar 2021;10(3):1391-1400. doi:10.21037/tau.2020.04.05. https://www.ncbi.nlm.nih.gov/pubmed/33850775 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8039587/pdf/tau-10-03-1391.pdf
- Ghafouri-Fard S, Shoorei H, Abak A, et al. Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. Oct 2021;142:112040. doi:10.1016/j.biopha.2021.112040. https://www.ncbi.nlm.nih.gov/pubmed/34416630
- Aitken RJ, Baker MA. The Role of Genetics and Oxidative Stress in the Etiology of Male Infertility-A Unifying Hypothesis? Frontiers in endocrinology. 2020;11:581838. doi:10.3389/fendo.2020.581838. https://www.ncbi.nlm.nih.gov/pubmed/33101214 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7554587/pdf/fendo-11-581838.pdf
- Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. May 2015;193(5):1596-601. doi:10.1016/j.juro.2014.11.080. https://www.ncbi.nlm.nih.gov/pubmed/25463997
- Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertility and sterility. Sep 2013;100(3):681-5. doi:10.1016/j.fertnstert.2013.05.022. https://www.ncbi.nlm.nih.gov/pubmed/23790640 https://www.fertstert.org/article/S0015-0282(13)00631-6/pdf
- Behboudi-Gandevani S, Bidhendi-Yarandi R, Panahi MH, Vaismoradi M. A Systematic Review and Meta-Analysis of Male Infertility and the Subsequent Risk of Cancer. Frontiers in oncology. 2021;11:696702. doi:10.3389/fonc.2021.696702. https://www.ncbi.nlm.nih.gov/pubmed/34722244
- Boeri L, Ventimiglia E, Cazzaniga W, et al. Risk of health status worsening in primary infertile men: A prospective 10-year follow-up study. Andrology. Aug 9 2021;doi:10.1111/andr.13090. https://www.ncbi.nlm.nih.gov/pubmed/34369670 https://onlinelibrary.wiley.com/doi/10.1111/andr.13090
- Del Giudice F, Kasman AM, Li S, et al. Increased Mortality Among Men Diagnosed With Impaired Fertility: Analysis of US Claims Data. Urology. Jan 2021;147:143-149. doi:10.1016/j.urology.2020.07.087. https://www.ncbi.nlm.nih.gov/pubmed/33017614 https://www.goldjournal.net/article/S0090-4295(20)31180-8/fulltext
- Tharakan T, Luo R, Jayasena CN, Minhas S. Non-obstructive azoospermia: current and future perspectives. Fac Rev. 2021;10:7. doi:10.12703/r/10-7. https://www.ncbi.nlm.nih.gov/pubmed/33659925 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7894261/pdf/facrev-10-07.pdf
- Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and Nutritional Factors in Male (In)fertility-Underestimated Factors. J Clin Med. May 9 2020;9(5)doi:10.3390/jcm9051400. https://mdpi-res.com/d_attachment/jcm/jcm-09-01400/article_deploy/jcm-09-01400.pdf
- Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reproductive biology and endocrinology : RB&E. Nov 26 2018;16(1):115. doi:10.1186/s12958-018-0436-9. https://www.ncbi.nlm.nih.gov/pubmed/30474562
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6260894/pdf/12958_2018_Article_436.pdf - Simionescu G, Doroftei B, Maftei R, et al. The complex relationship between infertility and psychological distress (Review). Experimental and therapeutic medicine. Apr 2021;21(4):306. doi:10.3892/etm.2021.9737. https://www.ncbi.nlm.nih.gov/pubmed/33717249
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7885086/pdf/etm-21-04-09737.pdf - Bedford JM. Human spermatozoa and temperature: the elephant in the room. Biol Reprod. Oct 2015;93(4):97. doi:10.1095/biolreprod.115.130658. https://watermark.silverchair.com/biolreprod0097.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAuMwggLfBgkqhkiG9w0BBwagggLQMIICzAIBADCCAsUGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMKaMuTHuPh0kRVFEzAgEQgIIClq_Bi0gMmMLHp6kMt3RzvjynXYvgttTbt4PTWLp-D77NHbhpD2I3xYa4gPcdsAkxlTkeHUmP7m4gRUFN3u49o4WYeuTvh0Os5vn0z2NkqQDgnSxBJVH7q2tIYGuiFyxwpxovwToie8j4Pwz0oR_JqlzxaUyc0SL47LK_-74RlPtmv8tCC0XVXhiK0gofcnAJRBaLPlmoYuRI4ETMChkAXAV3-58_HletvRA2VPxEFtaF5dt4rZz9COJz_9w6PQewUqpkjmEXxa1EV5bzg6fAc7qspwLjMRA4_bX0KKNIWu65QTnEBNmdoYYpF9h8IfBoQq2StU6iv_VT5qs33jCGKr4HY9E99i7XYEctOXXOZADSakFGLCXu0Bjs_hNF0OPhNfBr3Gvs_Fz9L3MQ8E67pCCJoiyzj--r6gDODhuaTrRwe5xa8_txvAT4zY8qA6W2I0P4GtNXrg3rc7B9Lqz5-y_Y1zaQnnW2cDAMKxg89szQyi4k6Bzg6bjzy6S6JIsfvaVALmxI0WfDHjIhTfv1YQmGbiz9qfCK-ABke5FLsWU2OcTphsPJ5SRIQqlnB_LqGDZaMy2Xx8D4hZVeUoGIPZcuPWaiz7hxKC7gzNfzkAf6EscOHStDnnw_f7dYgsZWgV2n3VZVd9u0lFna4EzP6nBjvpSy_JnhErtHwH_SzmsBJ4LAjH7HduV3qVaK-jlNyaQcH-Um2TuoUtmUpXuogeMKZJCDLU8hdKPBzve31d9dRZRDAuJCCNHEv-i_5lEkP3QPCMJy7zkr1Tm5lywgqLpNX85FvEYDanhIMIk_bA9OxRRIOSRo0B4QbxXCGMCKlJzVOAnlIGs2gJmS-QoChwaWdp_cA8yYwj8PuEOejySVCE2Jxc3g
- Mínguez-Alarcón L, Gaskins AJ, Chiu YH, et al. Type of underwear worn and markers of testicular function among men attending a fertility center. Human reproduction (Oxford, England). Sep 1 2018;33(9):1749-1756. doi:10.1093/humrep/dey259. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6530653/pdf/dey259.pdf
- Kaya C, Aykaç A, Kaya Y, Taş M. The effect of modifiable lifestyle factors on semen quality. Rev Int Androl. Oct-Dec 2020;18(4):151-158. doi:10.1016/j.androl.2019.09.001.
- Sapra KJ, Eisenberg ML, Kim S, Chen Z, Buck Louis GM. Choice of underwear and male fecundity in a preconception cohort of couples. Andrology. May 2016;4(3):500-8. doi:10.1111/andr.12163. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12163?download=true
- Gill K, Jakubik J, Kups M, et al. The impact of sedentary work on sperm nuclear DNA integrity. Folia Histochem Cytobiol. 2019;57(1):15-22. doi:10.5603/FHC.a2019.0002.
- Mieusset R, Bengoudifa B, Bujan L. Effect of posture and clothing on scrotal temperature in fertile men. Journal of andrology. Jan-Feb 2007;28(1):170-5. doi:10.2164/jandrol.106.000646. https://onlinelibrary.wiley.com/doi/pdfdirect/10.2164/jandrol.106.000646?download=true
- Semet M, Paci M, Saïas-Magnan J, et al. The impact of drugs on male fertility: a review. Andrology. Jul 2017;5(4):640-663. doi:10.1111/andr.12366. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12366?download=true
- Ilnitsky S, Van Uum S. Marijuana and fertility. CMAJ. Jun 10 2019;191(23):E638. doi:10.1503/cmaj.181577. https://www.ncbi.nlm.nih.gov/pubmed/31182459
- Gundersen TD, Jørgensen N, Andersson AM, et al. Association Between Use of Marijuana and Male Reproductive Hormones and Semen Quality: A Study Among 1,215 Healthy Young Men. American journal of epidemiology. Sep 15 2015;182(6):473-81. doi:10.1093/aje/kwv135.
- du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. Nov 2015;32(11):1575-88. doi:10.1007/s10815-015-0553-8. https://www.ncbi.nlm.nih.gov/pubmed/26277482 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651943/pdf/10815_2015_Article_553.pdf
- Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW. Cannabis and Male Fertility: A Systematic Review. J Urol. Oct 2019;202(4):674-681. doi:10.1097/JU.0000000000000248. https://www.ncbi.nlm.nih.gov/pubmed/30916627
- Panara K, Masterson JM, Savio LF, Ramasamy R. Adverse Effects of Common Sports and Recreational Activities on Male Reproduction. European urology focus. Nov 2019;5(6):1146-1151. doi:10.1016/j.euf.2018.04.013. https://www.ncbi.nlm.nih.gov/pubmed/29731401 https://www.eu-focus.europeanurology.com/article/S2405-4569(18)30103-2/pdf
- Rajanahally S, Raheem O, Rogers M, et al. The relationship between cannabis and male infertility, sexual health, and neoplasm: a systematic review. Andrology. Mar 2019;7(2):139-147. doi:10.1111/andr.12585. https://www.ncbi.nlm.nih.gov/pubmed/30767424
- Mongioi L, Calogero AE, Vicari E, et al. The role of carnitine in male infertility. Andrology. Sep 2016;4(5):800-7. doi:10.1111/andr.12191. https://www.ncbi.nlm.nih.gov/pubmed/27152678 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12191?download=true
- Sun LL, Wan XX, Zhang Y, et al. [L-carnitine improves sperm acrosin activity in male infertility patients]. Zhonghua Nan Ke Xue. Dec 2018;24(12):1064-1068. https://www.ncbi.nlm.nih.gov/pubmed/32212483
- Cheng JB, Zhu J, Ni F, Jiang H. [L-carnitine combined with coenzyme Q10 for idiopathic oligoasthenozoospermia: A double-blind randomized controlled trial]. Zhonghua Nan Ke Xue. Janurary 2018;24(1):33-38. https://www.ncbi.nlm.nih.gov/pubmed/30157357
- Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: A systematic review and meta-analysis. Andrologia. Mar 2020;52(2):e13470. doi:10.1111/and.13470. https://www.ncbi.nlm.nih.gov/pubmed/31701550 https://onlinelibrary.wiley.com/doi/10.1111/and.13470
- Mathieu d'Argent E, Ravel C, Rousseau A, et al. High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial). J Clin Med. Apr 26 2021;10(9)doi:10.3390/jcm10091876. https://mdpi-res.com/d_attachment/jcm/jcm-10-01876/article_deploy/jcm-10-01876-v2.pdf
- Bahmyari R, Ariafar A, Sayadi M, Hossieni S, Azima S. The Effect of Daily Intake of Selenium, Vitamin E and Folic Acid on Sperm Parameters in Males with Idiopathic Infertility: A Single-Blind Randomized Controlled Clinical Trial. Int J Fertil Steril. Jan 2021;15(1):8-14. doi:10.22074/ijfs.2021.6236. https://www.ncbi.nlm.nih.gov/pubmed/33497041
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7838762/pdf/Int-J-Fertil-Steril-15-8.pdf - Schisterman EF, Sjaarda LA, Clemons T, et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA. Jan 7 2020;323(1):35-48. doi:10.1001/jama.2019.18714. https://www.ncbi.nlm.nih.gov/pubmed/31910279
https://jamanetwork.com/journals/jama/articlepdf/2758450/jama_schisterman_2020_oi_190134.pdf - Hong HH, Hu Y, Yu XQ, et al. Associations of C677T polymorphism in methylenetetrahydrofolate reductase (MTHFR) gene with male infertility risk: A meta-analysis. European journal of obstetrics, gynecology, and reproductive biology. May 2017;212:101-109. doi:10.1016/j.ejogrb.2017.03.004. https://www.ncbi.nlm.nih.gov/pubmed/28363185
https://www.ejog.org/article/S0301-2115(17)30106-9/fulltext - Xie C, Ping P, Ma Y, Wu Z, Chen X. Correlation between methylenetetrahydrofolate reductase gene polymorphism and oligoasthenospermia and the effects of folic acid supplementation on semen quality. Transl Androl Urol. Dec 2019;8(6):678-685. doi:10.21037/tau.2019.11.17. https://www.ncbi.nlm.nih.gov/pubmed/32038964
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6987591/pdf/tau-08-06-678.pdf - Najafipour R, Moghbelinejad S, Aleyasin A, Jalilvand A. Effect of B9 and B12 vitamin intake on semen parameters and fertility of men with MTHFR polymorphisms. Andrology. Jul 2017;5(4):704-710. doi:10.1111/andr.12351. https://www.ncbi.nlm.nih.gov/pubmed/28440964
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12351?download=true - Huang WJ, Lu XL, Li JT, Zhang JM. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: a double-blind, randomized, placebo-controlled trial. Andrology. Jan 2020;8(1):110-116. doi:10.1111/andr.12652. https://www.ncbi.nlm.nih.gov/pubmed/31127676
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12652?download=true - Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica; the fate of foreign compounds in biological systems. May 2014;44(5):480-8. doi:10.3109/00498254.2013.845705. https://www.tandfonline.com/doi/full/10.3109/00498254.2013.845705
- Salvio G, Cutini M, Ciarloni A, Giovannini L, Perrone M, Balercia G. Coenzyme Q10 and Male Infertility: A Systematic Review. Antioxidants (Basel, Switzerland). May 30 2021;10(6)doi:10.3390/antiox10060874. https://www.ncbi.nlm.nih.gov/pubmed/34070761
https://mdpi-res.com/d_attachment/antioxidants/antioxidants-10-00874/article_deploy/antioxidants-10-00874-v2.pdf - Banihani SA. Effect of Coenzyme Q10 Supplementation on Testosterone. Biomolecules. Dec 13 2018;8(4)doi:10.3390/biom8040172. https://www.ncbi.nlm.nih.gov/pubmed/30551653 https://mdpi-res.com/d_attachment/biomolecules/biomolecules-08-00172/article_deploy/biomolecules-08-00172.pdf
- Lafuente R, González-Comadrán M, Solà I, et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. Sep 2013;30(9):1147-56. doi:10.1007/s10815-013-0047-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3800531/pdf/10815_2013_Article_47.pdf
- Vishvkarma R, Alahmar AT, Gupta G, Rajender S. Coenzyme Q10 effect on semen parameters: Profound or meagre? Andrologia. Jul 2020;52(6):e13570. doi:10.1111/and.13570. https://www.ncbi.nlm.nih.gov/pubmed/32271472 https://onlinelibrary.wiley.com/doi/10.1111/and.13570
- Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. Aug 2012;188(2):526-31. doi:10.1016/j.juro.2012.03.131. https://www.ncbi.nlm.nih.gov/pubmed/22704112
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. Jul 2009;182(1):237-48. doi:10.1016/j.juro.2009.02.121. https://www.ncbi.nlm.nih.gov/pubmed/19447425
- Nadjarzadeh A, Shidfar F, Amirjannati N, et al. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. Mar 2014;46(2):177-83. doi:10.1111/and.12062. https://www.ncbi.nlm.nih.gov/pubmed/23289958
https://onlinelibrary.wiley.com/doi/10.1111/and.12062 - Alahmar AT, Sengupta P. Impact of Coenzyme Q10 and Selenium on Seminal Fluid Parameters and Antioxidant Status in Men with Idiopathic Infertility. Biol Trace Elem Res. Apr 2021;199(4):1246-1252. doi:10.1007/s12011-020-02251-3. https://www.ncbi.nlm.nih.gov/pubmed/32572802 https://link.springer.com/content/pdf/10.1007/s12011-020-02251-3.pdf
- Vazquez-Levin MH, Verón GL. Myo-inositol in health and disease: its impact on semen parameters and male fertility. Andrology. Mar 2020;8(2):277-298. doi:10.1111/andr.12718. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12718?download=true
- Murgia F, Corda V, Serrenti M, et al. Seminal Fluid Metabolomic Markers of Oligozoospermic Infertility in Humans. Metabolites. Feb 11 2020;10(2)doi:10.3390/metabo10020064. https://www.ncbi.nlm.nih.gov/pubmed/32053951 https://mdpi-res.com/d_attachment/metabolites/metabolites-10-00064/article_deploy/metabolites-10-00064-v2.pdf
- Ghasemi A, Amjadi F, Masoumeh Ghazi Mirsaeed S, et al. The effect of Myo-inositol on sperm parameters and pregnancy rate in oligoasthenospermic men treated with IUI: A randomized clinical trial. Int J Reprod Biomed. Oct 2019;17(10):749-756. doi:10.18502/ijrm.v17i10.5296. https://www.ncbi.nlm.nih.gov/pubmed/31807723
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6844281/pdf/ijrb-17-749.pdf - Montanino Oliva M, Buonomo G, Carra MC, Lippa A, Lisi F. Myo-inositol impact on sperm motility in vagina and evaluation of its effects on foetal development. European review for medical and pharmacological sciences. Mar 2020;24(5):2704-2709. doi:10.26355/eurrev_202003_20540. https://www.ncbi.nlm.nih.gov/pubmed/32196621
- Montanino Oliva M, Poverini R, Lisi R, Carra MC, Lisi F. Treating Woman with Myo-Inositol Vaginal Suppositories Improves Partner's Sperm Motility and Fertility. International journal of endocrinology. 2016;2016:7621942. doi:10.1155/2016/7621942. https://www.ncbi.nlm.nih.gov/pubmed/27403162
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923529/pdf/IJE2016-7621942.pdf - Gulino FA, Leonardi E, Marilli I, et al. Effect of treatment with myo-inositol on semen parameters of patients undergoing an IVF cycle: in vivo study. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2016;32(1):65-8. doi:10.3109/09513590.2015.1080680. https://www.ncbi.nlm.nih.gov/pubmed/26361940 https://www.tandfonline.com/doi/full/10.3109/09513590.2015.1080680
- Calogero AE, Gullo G, La Vignera S, Condorelli RA, Vaiarelli A. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebo-controlled study. Andrology. May 2015;3(3):491-5. doi:10.1111/andr.12025. https://www.ncbi.nlm.nih.gov/pubmed/25854593
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12025?download=true - Vickram S, Rohini K, Srinivasan S, et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. International journal of molecular sciences. Feb 22 2021;22(4)doi:10.3390/ijms22042188. https://www.ncbi.nlm.nih.gov/pubmed/33671837 https://mdpi-res.com/d_attachment/ijms/ijms-22-02188/article_deploy/ijms-22-02188-v2.pdf
- Beigi Harchegani A, Dahan H, Tahmasbpour E, Bakhtiari Kaboutaraki H, Shahriary A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: the role of oxidative stress, inflammation and apoptosis. Hum Fertil (Camb). Apr 2020;23(1):5-16. doi:10.1080/14647273.2018.1494390. https://www.ncbi.nlm.nih.gov/pubmed/30129823
https://www.tandfonline.com/doi/full/10.1080/14647273.2018.1494390 - Zhao J, Dong X, Hu X, et al. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci Rep. Mar 2 2016;6:22386. doi:10.1038/srep22386. https://www.ncbi.nlm.nih.gov/pubmed/26932683 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4773819/pdf/srep22386.pdf
- Taravati A, Tohidi F. Association between seminal plasma zinc level and asthenozoospermia: a meta-analysis study. Andrologia. Aug 2016;48(6):646-53. doi:10.1111/and.12494. https://www.ncbi.nlm.nih.gov/pubmed/26541500 https://onlinelibrary.wiley.com/doi/10.1111/and.12494
- Allouche-Fitoussi D, Breitbart H. The Role of Zinc in Male Fertility. International journal of molecular sciences. Oct 21 2020;21(20)doi:10.3390/ijms21207796. https://www.ncbi.nlm.nih.gov/pubmed/33096823
- Omu AE, Dashti H, Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. European journal of obstetrics, gynecology, and reproductive biology. Aug 1998;79(2):179-84. doi:10.1016/s0301-2115(97)00262-5. https://www.ncbi.nlm.nih.gov/pubmed/9720838
https://www.ejog.org/article/S0301-2115(97)00262-5/fulltext - Irani M, Amirian M, Sadeghi R, Lez JL, Latifnejad Roudsari R. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol J. Aug 29 2017;14(5):4069-4078. https://www.ncbi.nlm.nih.gov/pubmed/28853101
https://journals.sbmu.ac.ir/urolj/index.php/uj/article/download/3772/1291 - Azizollahi G, Azizollahi S, Babaei H, Kianinejad M, Baneshi MR, Nematollahi-mahani SN. Effects of supplement therapy on sperm parameters, protamine content and acrosomal integrity of varicocelectomized subjects. J Assist Reprod Genet. Apr 2013;30(4):593-9. doi:10.1007/s10815-013-9961-9. https://www.ncbi.nlm.nih.gov/pubmed/23435530
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3644122/pdf/10815_2013_Article_9961.pdf - Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. International journal of environmental research and public health. Apr 2010;7(4):1342-65. doi:10.3390/ijerph7041342. https://mdpi-res.com/d_attachment/ijerph/ijerph-07-01342/article_deploy/ijerph-07-01342.pdf
- Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. Br J Urol. Jul 1998;82(1):76-80. doi:10.1046/j.1464-410x.1998.00683.x. https://www.ncbi.nlm.nih.gov/pubmed/9698665 https://bjui-journals.onlinelibrary.wiley.com/doi/abs/10.1046/j.1464-410x.1998.00683.x?sid=nlm%3Apubmed
- Hawkes WC, Alkan Z, Wong K. Selenium supplementation does not affect testicular selenium status or semen quality in North American men. Journal of andrology. Sep-Oct 2009;30(5):525-33. doi:10.2164/jandrol.108.006940. https://www.ncbi.nlm.nih.gov/pubmed/19342701
https://onlinelibrary.wiley.com/doi/pdfdirect/10.2164/jandrol.108.006940?download=true - Schwalfenberg GK. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J Nutr Metab. 2021;2021:9949453. doi:10.1155/2021/9949453. https://www.ncbi.nlm.nih.gov/pubmed/34221501 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8211525/pdf/jnme2021-9949453.pdf
- Zhou Z, Cui Y, Zhang X, Zhang Y. The role of N-acetyl-cysteine (NAC) orally daily on the sperm parameters and serum hormones in idiopathic infertile men: A systematic review and meta-analysis of randomised controlled trials. Andrologia. Mar 2021;53(2):e13953. doi:10.1111/and.13953. https://www.ncbi.nlm.nih.gov/pubmed/33405232
https://onlinelibrary.wiley.com/doi/10.1111/and.13953 - Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. Jul 2009;74(1):73-6. doi:10.1016/j.urology.2009.02.034. https://www.ncbi.nlm.nih.gov/pubmed/19428083 https://www.goldjournal.net/article/S0090-4295(09)00301-X/fulltext
- Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. Feb 2009;181(2):741-51. doi:10.1016/j.juro.2008.10.015. https://www.ncbi.nlm.nih.gov/pubmed/19091331
- Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reproductive biology and endocrinology : RB&E. Feb 16 2019;17(1):24. doi:10.1186/s12958-019-0468-9. https://www.ncbi.nlm.nih.gov/pubmed/30771790
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6377938/pdf/12958_2019_Article_468.pdf - Zareba P, Colaci DS, Afeiche M, et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertility and sterility. Dec 2013;100(6):1572-9. doi:10.1016/j.fertnstert.2013.08.032. https://www.fertstert.org/article/S0015-0282(13)02998-1/pdf
- Ghyasvand T, Goodarzi MT, Amiri I, Karimi J, Ghorbani M. Serum levels of lycopene, beta-carotene, and retinol and their correlation with sperm DNA damage in normospermic and infertile men. Int J Reprod Biomed. Dec 2015;13(12):787-92. https://www.ncbi.nlm.nih.gov/pubmed/27141539 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4827510/pdf/ijrb-13-787.pdf
- Filipcikova R, Oborna I, Brezinova J, et al. Lycopene improves the distorted ratio between AA/DHA in the seminal plasma of infertile males and increases the likelihood of successful pregnancy. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. Mar 2015;159(1):77-82. doi:10.5507/bp.2013.007. https://www.ncbi.nlm.nih.gov/pubmed/23446211 http://biomed.papers.upol.cz/pdfs/bio/2015/01/11.pdf
- Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility--a preliminary report. International urology and nephrology. 2002;34(3):369-72. doi:10.1023/a:1024483520560. https://www.ncbi.nlm.nih.gov/pubmed/12899230 https://link.springer.com/content/pdf/10.1023/A:1024483520560.pdf
- Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. Dec 2019;33(12):3203-3211. doi:10.1002/ptr.6493. https://www.ncbi.nlm.nih.gov/pubmed/31468596
https://onlinelibrary.wiley.com/doi/10.1002/ptr.6493 - Yamamoto Y, Aizawa K, Mieno M, et al. The effects of tomato juice on male infertility. Asia Pac J Clin Nutr. Jan 2017;26(1):65-71. doi:10.6133/apjcn.102015.17. https://www.ncbi.nlm.nih.gov/pubmed/28049263
- Williams EA, Parker M, Robinson A, Pitt S, Pacey AA. A randomized placebo-controlled trial to investigate the effect of lactolycopene on semen quality in healthy males. European journal of nutrition. Mar 2020;59(2):825-833. doi:10.1007/s00394-019-02091-5. https://www.ncbi.nlm.nih.gov/pubmed/31591650
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7058571/pdf/394_2019_Article_2091.pdf - Zerbinati C, Caponecchia L, Rago R, et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology. Nov 2016;4(6):1094-1101. doi:10.1111/andr.12236. https://www.ncbi.nlm.nih.gov/pubmed/27673576 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12236?download=true
- Collodel G, Moretti E, Noto D, Iacoponi F, Signorini C. Fatty Acid Profile and Metabolism Are Related to Human Sperm Parameters and Are Relevant in Idiopathic Infertility and Varicocele. Mediators Inflamm. 2020;2020:3640450. doi:10.1155/2020/3640450. https://www.ncbi.nlm.nih.gov/pubmed/32934603
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7479464/pdf/MI2020-3640450.pdf - Craig LB, Brush RS, Sullivan MT, Zavy MT, Agbaga MP, Anderson RE. Decreased very long chain polyunsaturated fatty acids in sperm correlates with sperm quantity and quality. J Assist Reprod Genet. Jul 2019;36(7):1379-1385. doi:10.1007/s10815-019-01464-3. https://www.ncbi.nlm.nih.gov/pubmed/31073727
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6642247/pdf/10815_2019_Article_1464.pdf - Tang LX, Yuan DJ, Wang QL, et al. Association of decreased spermatozoa omega-3 fatty acid levels and increased oxidative DNA damage with varicocele in infertile men: a case control study. Reprod Fertil Dev. Apr 2016;28(5):648-54. doi:10.1071/RD14276. https://www.ncbi.nlm.nih.gov/pubmed/25405715
https://www.publish.csiro.au/RD/RD14276 - Andersen JM, Ronning PO, Herning H, Bekken SD, Haugen TB, Witczak O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology. Sep 2016;4(5):857-65. doi:10.1111/andr.12227. https://www.ncbi.nlm.nih.gov/pubmed/27371336 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12227?download=true
- Falsig AL, Gleerup CS, Knudsen UB. The influence of omega-3 fatty acids on semen quality markers: a systematic PRISMA review. Andrology. Nov 2019;7(6):794-803. doi:10.1111/andr.12649. https://www.ncbi.nlm.nih.gov/pubmed/31116515 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12649?download=true
- Hosseini B, Nourmohamadi M, Hajipour S, et al. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. Journal of dietary supplements. 2019;16(2):245-256. doi:10.1080/19390211.2018.1431753. https://www.ncbi.nlm.nih.gov/pubmed/29451828
https://www.tandfonline.com/doi/full/10.1080/19390211.2018.1431753 - Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. Feb 2011;43(1):38-47. doi:10.1111/j.1439-0272.2009.01013.x. https://www.ncbi.nlm.nih.gov/pubmed/21219381 https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0272.2009.01013.x
- Martinez-Soto JC, Domingo JC, Cordobilla B, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med. Dec 2016;62(6):387-395. doi:10.1080/19396368.2016.1246623. https://www.ncbi.nlm.nih.gov/pubmed/27792396 https://www.tandfonline.com/doi/pdf/10.1080/19396368.2016.1246623?needAccess=true
- Vinogradov IV, Gamidov SI, Gabliya MY, et al. [Docosahexaenoic acid in the treatment of idiopathic male infertility]. Urologiia (Moscow, Russia : 1999). Apr 2019;(1):78-83. https://www.ncbi.nlm.nih.gov/pubmed/31184022
- Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids. Feb 2000;35(2):149-54. doi:10.1007/BF02664764. https://www.ncbi.nlm.nih.gov/pubmed/10757545 https://aocs.onlinelibrary.wiley.com/doi/abs/10.1007/BF02664764?sid=nlm%3Apubmed
- Eslamian G, Amirjannati N, Noori N, Sadeghi MR, Hekmatdoost A. Effects of coadministration of DHA and vitamin E on spermatogram, seminal oxidative stress, and sperm phospholipids in asthenozoospermic men: a randomized controlled trial. Am J Clin Nutr. Sep 1 2020;112(3):707-719. doi:10.1093/ajcn/nqaa124. https://www.ncbi.nlm.nih.gov/pubmed/32453396 https://watermark.silverchair.com/nqaa124.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAsUwggLBBgkqhkiG9w0BBwagggKyMIICrgIBADCCAqcGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMnXFZoB9W_mLLHYhHAgEQgIICePVNmr7p-NFj5jglvM7RF5PqN_Bf-bbPl78vydCnktf-iduJUpN0Ux5mPP4gHfRL1dvwEyk8CBlbYaF5tMaA22jptW7LZ0NiHBvWnP93RJHk-MOBDTinW7eQ7581IJq65d-JB31k04Oi6_DH4B-7iUigq4DnuQu7QqvHE12QUFwXKXaJ1LeO-5qyq5mtOwry3Yz_-tQQQvubEt4DMA0x4FhOHY7WCBN_IxNR80tLKABMs_ijz__mz4rSSP7HYODoS-d3c41Cdi7SuP0pJXm48EWsJQlqSx85EJZ3MNnKUkk9ub_iFJ9dAaJ2nn8d_MrOsZDYcsTlj6ZLJIUjmUjAbmyhTVkwN72Xeyg3RPE7VFEyqT987xKrfXO7YtOofK7ZWuzC1gX8E-8vGqjuCdA9KgIv-lLfYCExkASyFldCXRJTAFYEcFM8lkObkds07TdiiVXMXuZ-3dLe2wSO_rsHEIjw03BcCf7pT4G1rVQTSp1l3cvlMb7gH6zTGUmEfqVTJGSAlfEff8SouSX-SkICdehDxW_RKtk2suPa-3dOFQ6BF2RiQk9XpTjFyd110uqUW_alg81L2V5NQ83mHsl6B51ib29M5vJFZYozEoCpZXUjoegHc-ILE9iAPV2kRapa2cMs1ODveoFwBAEIDyBlOAIjAzFQUMclsppWnOWtWyzB8onzZDOT74_IDfrre1yhFr-kIvqj0dkZ_N0_HK8Skz-2TGfdR8qgzBwB0ggOJ3MibS2BM_kLMZFw2q4w3g4qKx7QK-QhgbG7-qINka13oYSbdYywpjj6kuXwENd5PawqAtMCzfO5Ps8YIGaCoYhNGVKGr-oxlAHV
- Wen JC. The Role of Vitamin E in the Treatment of Male Infertility. Nutrition Bytes. 2006;11(1)https://escholarship.org/uc/item/1s2485fw
- Adami LNG, Belardin LB, Lima BT, et al. Effect of in vitro vitamin E (alpha-tocopherol) supplementation in human spermatozoon submitted to oxidative stress. Andrologia. Feb 2 2018;doi:10.1111/and.12959. https://www.ncbi.nlm.nih.gov/pubmed/29392756 https://onlinelibrary.wiley.com/doi/10.1111/and.12959
- ElSheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: A prospective, randomized trial. Andrology. Sep 2015;3(5):864-7. doi:10.1111/andr.12086. https://www.ncbi.nlm.nih.gov/pubmed/26235968
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12086?download=true - Chen XF, Li Z, Ping P, Dai JC, Zhang FB, Shang XJ. [Efficacy of natural vitamin E on oligospermia and asthenospermia: a prospective multi-centered randomized controlled study of 106 cases]. Zhonghua Nan Ke Xue. May 2012;18(5):428-31. https://www.ncbi.nlm.nih.gov/pubmed/22741442
- Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. International journal of general medicine. Jan 23 2011;4:99-104. doi:10.2147/IJGM.S16275. https://www.ncbi.nlm.nih.gov/pubmed/21403799 https://www.dovepress.com/getfile.php?fileID=8612
- Sabeti P, Pourmasumi S, Fagheirelahee N. Effect of Selenium and Vitamin E on the Level of Sperm HSPA2+, Intracellular Superoxide Anion and Chromatin Integrity in Idiopathic Asthenoteratozoospermia: A Double-Blind, Randomized, Placebo- Controlled Trial. Urol J. Sep 13 2021;doi:10.22037/uj.v18i.6325. https://www.ncbi.nlm.nih.gov/pubmed/34516655
https://journals.sbmu.ac.ir/urolj/index.php/uj/article/download/6325/4103 - Ener K, Aldemir M, Isik E, et al. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: a randomised controlled study. Andrologia. Sep 2016;48(7):829-34. doi:10.1111/and.12521. https://www.ncbi.nlm.nih.gov/pubmed/26780969 https://onlinelibrary.wiley.com/doi/10.1111/and.12521
- Tandon N, Yadav SS. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. Journal of ethnopharmacology. Jun 12 2020;255:112768. doi:10.1016/j.jep.2020.112768. https://www.ncbi.nlm.nih.gov/pubmed/32201301
- Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iranian journal of basic medical sciences. Dec 2020;23(12):1501-1526. doi:10.22038/IJBMS.2020.44254.10378. https://www.ncbi.nlm.nih.gov/pubmed/33489024
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7811807/pdf/IJBMS-23-1501.pdf - Durg S, Shivaram SB, Bavage S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine. Nov 15 2018;50:247-256. doi:10.1016/j.phymed.2017.11.011. https://www.ncbi.nlm.nih.gov/pubmed/30466985
- Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online. Mar 2018;36(3):311-326. doi:10.1016/j.rbmo.2017.11.007. https://www.ncbi.nlm.nih.gov/pubmed/29277366 https://www.rbmojournal.com/article/S1472-6483(17)30625-9/pdf
- Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:571420. doi:10.1155/2013/571420. https://www.ncbi.nlm.nih.gov/pubmed/24371462 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3863556/pdf/ECAM2013-571420.pdf
- Ahmad MK, Mahdi AA, Shukla KK, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertility and sterility. Aug 2010;94(3):989-96. doi:10.1016/j.fertnstert.2009.04.046. https://www.ncbi.nlm.nih.gov/pubmed/19501822 https://www.fertstert.org/article/S0015-0282(09)01014-0/pdf
- Mahdi AA, Shukla KK, Ahmad MK, et al. Withania somnifera Improves Semen Quality in Stress-Related Male Fertility. Evidence-based complementary and alternative medicine : eCAM. Sep 29 2009;2011doi:10.1093/ecam/nep138. https://www.ncbi.nlm.nih.gov/pubmed/19789214 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3136684/pdf/ECAM2011-576962.pdf
- Leung KW, Wong AS. Ginseng and male reproductive function. Spermatogenesis. Jul 1 2013;3(3):e26391. doi:10.4161/spmg.26391. https://www.ncbi.nlm.nih.gov/pubmed/24381805 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3861174/pdf/spmg-3-e26391.pdf
- Park HJ, Choe S, Park NC. Effects of Korean red ginseng on semen parameters in male infertility patients: A randomized, placebo-controlled, double-blind clinical study. Chinese journal of integrative medicine. Jul 2016;22(7):490-5. doi:10.1007/s11655-015-2139-9. https://www.ncbi.nlm.nih.gov/pubmed/25967606
https://link.springer.com/content/pdf/10.1007/s11655-015-2139-9.pdf - Cai T, Wagenlehner FM, Mazzoli S, et al. Semen quality in patients with Chlamydia trachomatis genital infection treated concurrently with prulifloxacin and a phytotherapeutic agent. Journal of andrology. Jul-Aug 2012;33(4):615-23. doi:10.2164/jandrol.111.013961. https://www.ncbi.nlm.nih.gov/pubmed/21979301 https://onlinelibrary.wiley.com/doi/pdfdirect/10.2164/jandrol.111.013961?download=true
- Frungieri MB, Calandra RS, Rossi SP. Local Actions of Melatonin in Somatic Cells of the Testis. International journal of molecular sciences. May 31 2017;18(6)doi:10.3390/ijms18061170. https://www.ncbi.nlm.nih.gov/pubmed/28561756 https://mdpi-res.com/d_attachment/ijms/ijms-18-01170/article_deploy/ijms-18-01170-v2.pdf
- Kratz EM, Piwowar A, Zeman M, Stebelová K, Thalhammer T. Decreased melatonin levels and increased levels of advanced oxidation protein products in the seminal plasma are related to male infertility. Reprod Fertil Dev. Mar 2016;28(4):507-15. doi:10.1071/rd14165. https://www.publish.csiro.au/RD/RD14165
- Sciarra F, Franceschini E, Campolo F, et al. Disruption of Circadian Rhythms: A Crucial Factor in the Etiology of Infertility. International journal of molecular sciences. May 30 2020;21(11)doi:10.3390/ijms21113943. https://www.ncbi.nlm.nih.gov/pubmed/32486326 https://mdpi-res.com/d_attachment/ijms/ijms-21-03943/article_deploy/ijms-21-03943-v2.pdf
- Wahl S, Engelhardt M, Schaupp P, Lappe C, Ivanov IV. The inner clock-Blue light sets the human rhythm. J Biophotonics. Dec 2019;12(12):e201900102. doi:10.1002/jbio.201900102. https://www.ncbi.nlm.nih.gov/pubmed/31433569 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7065627/pdf/JBIO-12-e201900102.pdf
- Green A, Barak S, Shine L, Kahane A, Dagan Y. Exposure by males to light emitted from media devices at night is linked with decline of sperm quality and correlated with sleep quality measures. Chronobiology international. Mar 2020;37(3):414-424. doi:10.1080/07420528.2020.1727918. https://www.ncbi.nlm.nih.gov/pubmed/32126861
https://www.tandfonline.com/doi/full/10.1080/07420528.2020.1727918 - Deng N, Kohn TP, Lipshultz LI, Pastuszak AW. The Relationship Between Shift Work and Men's Health. Sexual medicine reviews. Jul 2018;6(3):446-456. doi:10.1016/j.sxmr.2017.11.009. https://www.ncbi.nlm.nih.gov/pubmed/29371140
- Malmir M, Naderi Noreini S, Ghafarizadeh A, Faraji T, Asali Z. Ameliorative effect of melatonin on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the idiopathic asthenoteratospermic men: In vitro. Andrologia. Mar 2021;53(2):e13944. doi:10.1111/and.13944. https://www.ncbi.nlm.nih.gov/pubmed/33368491
https://onlinelibrary.wiley.com/doi/10.1111/and.13944 - Lu XL, Liu JJ, Li JT, Yang QA, Zhang JM. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: A placebo-controlled, double-blind trial. Andrologia. Aug 2018;50(6):e13033. doi:10.1111/and.13033. https://www.ncbi.nlm.nih.gov/pubmed/29740842 https://onlinelibrary.wiley.com/doi/10.1111/and.13033
- Bejarano I, Monllor F, Marchena AM, et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. Journal of pineal research. Oct 2014;57(3):333-9. doi:10.1111/jpi.12172.
- Roseff SJ. Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med. Oct 2002;47(10):821-4. https://www.ncbi.nlm.nih.gov/pubmed/12418064
- Stanislavov R, Rohdewald P. Sperm quality in men is improved by supplementation with a combination of L-arginine, L-citrullin, roburins and Pycnogenol(R). Minerva urologica e nefrologica = The Italian journal of urology and nephrology. Dec 2014;66(4):217-23. https://www.ncbi.nlm.nih.gov/pubmed/25531191
- Kobori Y, Suzuki K, Iwahata T, et al. Improvement of seminal quality and sexual function of men with oligoasthenoteratozoospermia syndrome following supplementation with L-arginine and Pycnogenol(R). Arch Ital Urol Androl. Sep 30 2015;87(3):190-3. doi:10.4081/aiua.2015.3.190. https://www.ncbi.nlm.nih.gov/pubmed/26428638
https://www.pagepressjournals.org/index.php/aiua/article/download/aiua.2015.3.190/4834 - Stefanescu R, Tero-Vescan A, Negroiu A, Aurica E, Vari CE. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules. May 12 2020;10(5)doi:10.3390/biom10050752. https://www.ncbi.nlm.nih.gov/pubmed/32408715 https://mdpi-res.com/d_attachment/biomolecules/biomolecules-10-00752/article_deploy/biomolecules-10-00752-v2.pdf
- GamalEl Din SF. Role of Tribulus terrestris in Male Infertility: Is It Real or Fiction? Journal of dietary supplements. Nov 2 2018;15(6):1010-1013. doi:10.1080/19390211.2017.1402843. https://www.ncbi.nlm.nih.gov/pubmed/29261340 https://www.tandfonline.com/doi/full/10.1080/19390211.2017.1402843
- Sanagoo S, Sadeghzadeh Oskouei B, Gassab Abdollahi N, Salehi-Pourmehr H, Hazhir N, Farshbaf-Khalili A. Effect of Tribulus terrestris L. on sperm parameters in men with idiopathic infertility: A systematic review. Complementary therapies in medicine. Feb 2019;42:95-103. doi:10.1016/j.ctim.2018.09.015. https://www.ncbi.nlm.nih.gov/pubmed/30670288
- Salgado RM, Marques-Silva MH, Gonçalves E, Mathias AC, Aguiar JG, Wolff P. Effect of oral administration of Tribulus terrestris extract on semen quality and body fat index of infertile men. Andrologia. Jun 2017;49(5)doi:10.1111/and.12655. https://onlinelibrary.wiley.com/doi/10.1111/and.12655
- Roaiah MF, Elkhayat YI, Abd El Salam MA, Din SFG. Prospective Analysis on the Effect of Botanical Medicine (Tribulus terrestris) on Serum Testosterone Level and Semen Parameters in Males with Unexplained Infertility. Journal of dietary supplements. Jan 2 2017;14(1):25-31. doi:10.1080/19390211.2016.1188193. https://www.ncbi.nlm.nih.gov/pubmed/27337519 https://www.tandfonline.com/doi/full/10.1080/19390211.2016.1188193
- GamalEl Din SF, Abdel Salam MA, Mohamed MS, et al. Tribulus terrestris versus placebo in the treatment of erectile dysfunction and lower urinary tract symptoms in patients with late-onset hypogonadism: A placebo-controlled study. Urologia. May 2019;86(2):74-78. doi:10.1177/0391560318802160. https://www.ncbi.nlm.nih.gov/pubmed/30253697
https://journals.sagepub.com/doi/10.1177/0391560318802160?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed - Roaiah MF, El Khayat YI, GamalEl Din SF, Abd El Salam MA. Pilot Study on the Effect of Botanical Medicine (Tribulus terrestris) on Serum Testosterone Level and Erectile Function in Aging Males With Partial Androgen Deficiency (PADAM). Journal of sex & marital therapy. May 18 2016;42(4):297-301. doi:10.1080/0092623X.2015.1033579. https://www.ncbi.nlm.nih.gov/pubmed/25849625 https://www.tandfonline.com/doi/full/10.1080/0092623X.2015.1033579
- Santos CA, Jr., Reis LO, Destro-Saade R, Luiza-Reis A, Fregonesi A. Tribulus terrestris versus placebo in the treatment of erectile dysfunction: A prospective, randomized, double blind study. Actas urologicas espanolas. May 2014;38(4):244-8. doi:10.1016/j.acuro.2013.09.014. https://www.ncbi.nlm.nih.gov/pubmed/24630840
- Neychev VK, Mitev VI. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. Journal of ethnopharmacology. Oct 3 2005;101(1-3):319-23. doi:10.1016/j.jep.2005.05.017. https://www.ncbi.nlm.nih.gov/pubmed/15994038
- Kuchakulla M, Masterson T, Arora H, Kulandavelu S, Ramasamy R. Effect of nitroso-redox imbalance on male reproduction. Transl Androl Urol. Dec 2018;7(6):968-977. doi:10.21037/tau.2018.08.14. https://www.ncbi.nlm.nih.gov/pubmed/30505735 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6256041/pdf/tau-07-06-968.pdf
- Di Tucci C, Galati G, Mattei G, et al. The role of alpha lipoic acid in female and male infertility: a systematic review. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Jun 2021;37(6):497-505. doi:10.1080/09513590.2020.1843619. https://www.ncbi.nlm.nih.gov/pubmed/33345661
https://www.tandfonline.com/doi/full/10.1080/09513590.2020.1843619 - Abbasi B, Molavi N, Tavalaee M, Abbasi H, Nasr-Esfahani MH. Alpha-lipoic acid improves sperm motility in infertile men after varicocelectomy: a triple-blind randomized controlled trial. Reprod Biomed Online. Dec 2020;41(6):1084-1091. doi:10.1016/j.rbmo.2020.08.013. https://www.ncbi.nlm.nih.gov/pubmed/33032908 https://www.rbmojournal.com/article/S1472-6483(20)30446-6/fulltext
- Gamidov SI, Ovchinnikov RI, Popova AY. [Double-blind, randomized placebo-controlled study of efficiency and safety of complex acetyl-L-carnitine, L-carnitine fumarate and alpha-lipoic acid (Spermactin Forte) for treatment of male infertility]. Urologiia (Moscow, Russia : 1999). Sep 2019;(4):62-68. https://www.ncbi.nlm.nih.gov/pubmed/31535807
- Ibrahim IA, Shalaby AA, Abd Elaziz RT, Bahr HI. Chlorella vulgaris or Spirulina platensis mitigate lead acetate-induced testicular oxidative stress and apoptosis with regard to androgen receptor expression in rats. Environ Sci Pollut Res Int. Aug 2021;28(29):39126-39138. doi:10.1007/s11356-021-13411-w. https://www.ncbi.nlm.nih.gov/pubmed/33754266
https://link.springer.com/content/pdf/10.1007/s11356-021-13411-w.pdf - Modarresi R, Aminsharifi A, Foroughinia F. Impact of Spirulina Supplementation on Semen Parameters in Patients with Idiopathic Male Infertility: A Pilot Randomized Trial. Urol J. Feb 21 2019;16(1):78-82. doi:10.22037/uj.v0i0.4122. https://www.ncbi.nlm.nih.gov/pubmed/30033514 https://journals.sbmu.ac.ir/urolj/index.php/uj/article/download/4122/1615
- Carrasco-Gallardo C, Guzmán L, Maccioni RB. Shilajit: a natural phytocomplex with potential procognitive activity. Int J Alzheimers Dis. 2012;2012:674142. doi:10.1155/2012/674142. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3296184/pdf/IJAD2012-674142.pdf
- Mishra RK, Jain A, Singh SK. Profertility effects of Shilajit on cadmium-induced infertility in male mice. Andrologia. Oct 2018;50(8):e13064. doi:10.1111/and.13064. https://www.ncbi.nlm.nih.gov/pubmed/29947420 https://onlinelibrary.wiley.com/doi/10.1111/and.13064
- Biswas TK, Pandit S, Mondal S, et al. Clinical evaluation of spermatogenic activity of processed Shilajit in oligospermia. Andrologia. Feb 2010;42(1):48-56. doi:10.1111/j.1439-0272.2009.00956.x. https://www.ncbi.nlm.nih.gov/pubmed/20078516 https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0272.2009.00956.x
- Maleki-Saghooni N, Mirzaeii K, Hosseinzadeh H, Sadeghi R, Irani M. A systematic review and meta-analysis of clinical trials on saffron (Crocus sativus) effectiveness and safety on erectile dysfunction and semen parameters. Avicenna journal of phytomedicine. May-Jun 2018;8(3):198-209. https://www.ncbi.nlm.nih.gov/pubmed/29881706
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5987435/pdf/AJP-8-198.pdf - Heidary M, Vahhabi S, Reza Nejadi J, et al. Effect of saffron on semen parameters of infertile men. Urol J. Fall 2008;5(4):255-9. https://www.ncbi.nlm.nih.gov/pubmed/19101900
- Safarinejad MR, Shafiei N, Safarinejad S. A prospective double-blind randomized placebo-controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia. Phytother Res. Apr 2011;25(4):508-16. doi:10.1002/ptr.3294. https://www.ncbi.nlm.nih.gov/pubmed/20824894 https://onlinelibrary.wiley.com/doi/10.1002/ptr.3294
- Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. The Cochrane database of systematic reviews. Mar 14 2019;3(3):CD007411. doi:10.1002/14651858.CD007411.pub4. https://www.ncbi.nlm.nih.gov/pubmed/30866036 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6416049/pdf/CD007411.pdf
- Ali M, Martinez M, Parekh N. Are antioxidants a viable treatment option for male infertility? Andrologia. Feb 2021;53(1):e13644. doi:10.1111/and.13644. https://www.ncbi.nlm.nih.gov/pubmed/32427374
- Arafa M, Agarwal A, Majzoub A, et al. Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants (Basel, Switzerland). Mar 6 2020;9(3)doi:10.3390/antiox9030219. https://www.ncbi.nlm.nih.gov/pubmed/32155908
https://mdpi-res.com/d_attachment/antioxidants/antioxidants-09-00219/article_deploy/antioxidants-09-00219.pdf - Lipovac M, Bodner F, Imhof M, Chedraui P. Comparison of the effect of a combination of eight micronutrients versus a standard mono preparation on sperm parameters. Reproductive biology and endocrinology : RB&E. Dec 9 2016;14(1):84. doi:10.1186/s12958-016-0219-0. https://www.ncbi.nlm.nih.gov/pubmed/27938385 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5148870/pdf/12958_2016_Article_219.pdf
- Micic S, Lalic N, Djordjevic D, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia. Jul 2019;51(6):e13267. doi:10.1111/and.13267. https://www.ncbi.nlm.nih.gov/pubmed/30873633 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6850469/pdf/AND-51-na.pdf
- Kopets R, Kuibida I, Chernyavska I, et al. Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: A randomized clinical study. Andrology. Sep 2020;8(5):1184-1193. doi:10.1111/andr.12805. https://www.ncbi.nlm.nih.gov/pubmed/32330373 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/andr.12805?download=true
- Canepa P, Dal Lago A, De Leo C, et al. Combined treatment with myo-inositol, alpha-lipoic acid, folic acid and vitamins significantly improves sperm parameters of sub-fertile men: a multi-centric study. European review for medical and pharmacological sciences. Oct 2018;22(20):7078-7085. doi:10.26355/eurrev_201810_16180. https://www.ncbi.nlm.nih.gov/pubmed/30402876
- Dinkova A, Martinov D, Konova E. Efficacy of myo-inositol in the clinical management of patients with asthenozoospermia. European review for medical and pharmacological sciences. Jun 2017;21(2 Suppl):62-65. https://www.ncbi.nlm.nih.gov/pubmed/28724182
- Montanino Oliva M, Minutolo E, Lippa A, Iaconianni P, Vaiarelli A. Effect of Myoinositol and Antioxidants on Sperm Quality in Men with Metabolic Syndrome. International journal of endocrinology. 2016;2016:1674950. doi:10.1155/2016/1674950. https://www.ncbi.nlm.nih.gov/pubmed/27752262 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5056296/pdf/IJE2016-1674950.pdf
- Korosi T, Barta C, Rokob K, Torok T. Physiological Intra-Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo-inositol in oligoasthenoteratozoospermic men: results from a prospective, randomized controlled trial. European review for medical and pharmacological sciences. Jun 2017;21(2 Suppl):66-72. https://www.ncbi.nlm.nih.gov/pubmed/28724183
- Vanderhout SM, Rastegar Panah M, Garcia-Bailo B, et al. Nutrition, genetic variation and male fertility. Transl Androl Urol. Mar 2021;10(3):1410-1431. doi:10.21037/tau-20-592. https://www.ncbi.nlm.nih.gov/pubmed/33850777 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8039611/pdf/tau-10-03-1410.pdf
- Aoun A, Khoury VE, Malakieh R. Can Nutrition Help in the Treatment of Infertility? Prev Nutr Food Sci. Jun 30 2021;26(2):109-120. doi:10.3746/pnf.2021.26.2.109.
- Efrat M, Stein A, Pinkas H, Unger R, Birk R. Dietary patterns are positively associated with semen quality. Fertility and sterility. May 2018;109(5):809-816. doi:10.1016/j.fertnstert.2018.01.010. https://www.ncbi.nlm.nih.gov/pubmed/29778381 https://www.fertstert.org/article/S0015-0282(18)30010-4/pdf
- Caruso P, Caputo M, Cirillo P, et al. Effects of Mediterranean diet on semen parameters in healthy young adults: a randomized controlled trial. Minerva endocrinologica. Dec 2020;45(4):280-287. doi:10.23736/S0391-1977.20.03362-3. https://www.ncbi.nlm.nih.gov/pubmed/33478205
- Yao DF, Mills JN. Male infertility: lifestyle factors and holistic, complementary, and alternative therapies. Asian journal of andrology. May-Jun 2016;18(3):410-8. doi:10.4103/1008-682X.175779. https://www.ncbi.nlm.nih.gov/pubmed/26952957 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854092/pdf/AJA-18-410.pdf
- Tang Q, Pan F, Wu X, et al. Semen quality and cigarette smoking in a cohort of healthy fertile men. Environ Epidemiol. Aug 2019;3(4):e055. doi:10.1097/EE9.0000000000000055. https://www.ncbi.nlm.nih.gov/pubmed/31538136
- Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. Journal of andrology. Jul-Aug 2012;33(4):515-28. doi:10.2164/jandrol.110.011874. https://www.ncbi.nlm.nih.gov/pubmed/21799144 https://onlinelibrary.wiley.com/doi/pdfdirect/10.2164/jandrol.110.011874?download=true
- Sansone A, Di Dato C, de Angelis C, et al. Smoke, alcohol and drug addiction and male fertility. Reproductive biology and endocrinology : RB&E. Jan 15 2018;16(1):3. doi:10.1186/s12958-018-0320-7. https://www.ncbi.nlm.nih.gov/pubmed/29334961
- Kohn TP, Kohn JR, Haney NM, Pastuszak AW, Lipshultz LI. The effect of sleep on men's health. Transl Androl Urol. Mar 2020;9(Suppl 2):S178-S185. doi:10.21037/tau.2019.11.07. https://www.ncbi.nlm.nih.gov/pubmed/32257858 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108988/pdf/tau-09-S2-S178.pdf
- Kim E, Zhao Z, Rzasa JR, et al. Association of acute psychosocial stress with oxidative stress: Evidence from serum analysis. Redox biology. Sep 16 2021;47:102138. doi:10.1016/j.redox.2021.102138. https://www.ncbi.nlm.nih.gov/pubmed/34555595 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8458980/pdf/main.pdf
- Allen J, Caruncho HJ, Kalynchuk LE. Severe life stress, mitochondrial dysfunction, and depressive behavior: A pathophysiological and therapeutic perspective. Mitochondrion. Jan 2021;56:111-117. doi:10.1016/j.mito.2020.11.010. https://www.ncbi.nlm.nih.gov/pubmed/33220501
- Lalinde-Acevedo PC, Mayorga-Torres BJM, Agarwal A, et al. Physically Active Men Show Better Semen Parameters than Their Sedentary Counterparts. Int J Fertil Steril. Oct 2017;11(3):156-165. doi:10.22074/ijfs.2017.4881. https://www.ncbi.nlm.nih.gov/pubmed/28868837
- Rosety MA, Diaz AJ, Rosety JM, et al. Exercise improved semen quality and reproductive hormone levels in sedentary obese adults. Nutr Hosp. Jun 5 2017;34(3):603-607. doi:10.20960/nh.549. https://www.ncbi.nlm.nih.gov/pubmed/28627195
- Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. Mar 2018;16(1):10-20. doi:10.1016/j.aju.2017.12.004. https://www.ncbi.nlm.nih.gov/pubmed/29713532
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5922227/pdf/main.pdf - Gonzalez D, Narasimman M, Best JC, Ory J, Ramasamy R. Clinical Update on Home Testing for Male Fertility. World J Mens Health. Oct 2021;39(4):615-625. doi:10.5534/wjmh.200130. https://www.ncbi.nlm.nih.gov/pubmed/33474845 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8443999/pdf/wjmh-39-615.pdf
- Turner KA, Rambhatla A, Schon S, et al. Male Infertility is a Women's Health Issue-Research and Clinical Evaluation of Male Infertility Is Needed. Cells. Apr 16 2020;9(4)doi:10.3390/cells9040990. https://www.ncbi.nlm.nih.gov/pubmed/32316195 https://mdpi-res.com/d_attachment/cells/cells-09-00990/article_deploy/cells-09-00990-v2.pdf
- A SV, Dhama K, Chakraborty S, et al. Role of Antisperm Antibodies in Infertility, Pregnancy, and Potential forContraceptive and Antifertility Vaccine Designs: Research Progress and Pioneering Vision. Vaccines. Sep 16 2019;7(3)doi:10.3390/vaccines7030116. https://www.ncbi.nlm.nih.gov/pubmed/31527552 https://mdpi-res.com/d_attachment/vaccines/vaccines-07-00116/article_deploy/vaccines-07-00116-v2.pdf
- Andrade DL, Viana MC, Esteves SC. Differential Diagnosis of Azoospermia in Men with Infertility. J Clin Med. Jul 16 2021;10(14)doi:10.3390/jcm10143144. https://www.ncbi.nlm.nih.gov/pubmed/34300309 https://mdpi-res.com/d_attachment/jcm/jcm-10-03144/article_deploy/jcm-10-03144-v2.pdf
- Calogero AE, Condorelli RA, Russo GI, La Vignera S. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed Res Int. 2017;2017:4650182. doi:10.1155/2017/4650182. https://www.ncbi.nlm.nih.gov/pubmed/28164122 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5253172/pdf/BMRI2017-4650182.pdf
- Mowat A, Newton C, Boothroyd C, Demmers K, Fleming S. The effects of vaginal lubricants on sperm function: an in vitro analysis. J Assist Reprod Genet. Mar 2014;31(3):333-9. doi:10.1007/s10815-013-0168-x. https://www.ncbi.nlm.nih.gov/pubmed/24390681 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3947082/pdf/10815_2013_Article_168.pdf
- Sandhu RS, Wong TH, Kling CA, Chohan KR. In vitro effects of coital lubricants and synthetic and natural oils on sperm motility. Fertility and sterility. Apr 2014;101(4):941-4. doi:10.1016/j.fertnstert.2013.12.024. https://www.ncbi.nlm.nih.gov/pubmed/24462060 https://www.fertstert.org/article/S0015-0282(13)03456-0/pdf
- Ring JD, Lwin AA, Köhler TS. Current medical management of endocrine-related male infertility. Asian journal of andrology. May-Jun 2016;18(3):357-63. doi:10.4103/1008-682x.179252. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854080/pdf/AJA-18-357.pdf
- Tadros NN, Sabanegh ES. Empiric medical therapy with hormonal agents for idiopathic male infertility. Indian journal of urology : IJU : journal of the Urological Society of India. Jul-Sep 2017;33(3):194-198. doi:10.4103/iju.IJU_368_16. https://www.ncbi.nlm.nih.gov/pubmed/28717268 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5508429/pdf/IJU-33-194.pdf
- Ramasamy R, Masterson TA, Best JC, et al. Effect of Natesto on Reproductive Hormones, Semen Parameters and Hypogonadal Symptoms: A Single Center, Open Label, Single Arm Trial. J Urol. Sep 2020;204(3):557-563. doi:10.1097/JU.0000000000001078. https://www.ncbi.nlm.nih.gov/pubmed/32294396
- Gronski MA, Grober ED, Gottesman IS, Ormsby RW, Bryson N. Efficacy of Nasal Testosterone Gel (Natesto(®)) Stratified by Baseline Endogenous Testosterone Levels. Journal of the Endocrine Society. 2019;3(9):1652-1662. doi:10.1210/js.2019-00183. https://pubmed.ncbi.nlm.nih.gov/31428719 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6694041/
- Boeri L, Capogrosso P, Salonia A. Gonadotropin Treatment for the Male Hypogonadotropic Hypogonadism. Curr Pharm Des. 2021;27(24):2775-2783. doi:10.2174/1381612826666200523175806. https://www.ncbi.nlm.nih.gov/pubmed/32445446 https://www.eurekaselect.com/182236/article
- Wheeler KM, Sharma D, Kavoussi PK, Smith RP, Costabile R. Clomiphene Citrate for the Treatment of Hypogonadism. Sexual medicine reviews. Apr 2019;7(2):272-276. doi:10.1016/j.sxmr.2018.10.001. https://www.ncbi.nlm.nih.gov/pubmed/30522888
- Chanson P, Borson-Chazot F, Chabre O, Estour B. Drug treatment of hyperprolactinemia. Annales d'endocrinologie. Jun 2007;68(2-3):113-7. doi:10.1016/j.ando.2007.03.003. https://www.ncbi.nlm.nih.gov/pubmed/17532288
- Lu J, Cai L, Wu Z, et al. Surgery and Medical Treatment in Microprolactinoma: A Systematic Review and Meta-Analysis. International journal of endocrinology. 2021;2021:9930059. doi:10.1155/2021/9930059. https://www.ncbi.nlm.nih.gov/pubmed/34504526 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8423556/pdf/IJE2021-9930059.pdf
- Horowitz AJ, Smith T, Frey D. Sympathomimetics. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Mehta A, Sigman M. Management of the dry ejaculate: a systematic review of aspermia and retrograde ejaculation. Fertility and sterility. Nov 2015;104(5):1074-81. doi:10.1016/j.fertnstert.2015.09.024. https://www.ncbi.nlm.nih.gov/pubmed/26432530 https://www.fertstert.org/article/S0015-0282(15)01952-4/pdf
- Omu AE, al-Qattan F, Abdul Hamada B. Effect of low dose continuous corticosteroid therapy in men with antisperm antibodies on spermatozoal quality and conception rate. European journal of obstetrics, gynecology, and reproductive biology. Nov 1996;69(2):129-34. doi:10.1016/0301-2115(95)02539-1. https://www.ncbi.nlm.nih.gov/pubmed/8902446
https://www.ejog.org/article/0301-2115(95)02539-1/pdf - Taiyeb AM, Ridha-Albarzanchi MT, Taiyeb SM, et al. Improvement in pregnancy outcomes in couples with immunologically male infertility undergoing prednisolone treatment and conventional in vitro fertilization preceded by sperm penetration assay: a randomized controlled trial. Endocrine. Dec 2017;58(3):448-457. doi:10.1007/s12020-017-1446-7. https://www.ncbi.nlm.nih.gov/pubmed/29030775 https://link.springer.com/content/pdf/10.1007/s12020-017-1446-7.pdf
- Omu AE, Makhseed M, Mohammed AT, Munim RA. Characteristics of men and women with circulating antisperm antibodies in a combined infertility clinic in Kuwait. Arch Androl. Jul-Aug 1997;39(1):55-64. doi:10.3109/01485019708987902. https://www.ncbi.nlm.nih.gov/pubmed/9202834 https://www.tandfonline.com/doi/pdf/10.3109/01485019708987902?needAccess=true
- Casarini L, Crépieux P, Reiter E, et al. FSH for the Treatment of Male Infertility. International journal of molecular sciences. Mar 25 2020;21(7)doi:10.3390/ijms21072270. https://mdpi-res.com/d_attachment/ijms/ijms-21-02270/article_deploy/ijms-21-02270-v2.pdf
- Cannarella R, La Vignera S, Condorelli RA, Mongioi LM, Calogero AE. FSH dosage effect on conventional sperm parameters: a meta-analysis of randomized controlled studies. Asian journal of andrology. May-Jun 2020;22(3):309-316. doi:10.4103/aja.aja_42_19. https://www.ncbi.nlm.nih.gov/pubmed/31274479 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7275804/pdf/AJA-22-309.pdf
- Zhao N, Lu XL, Li JT, Zhang JM. Treatment of idiopathic oligozoospermia with combined human chorionic gonadotropin/human menopausal gonadotrophin: A randomised, double-blinded, placebo-controlled clinical study. Andrologia. Jul 2019;51(6):e13271. doi:10.1111/and.13271. https://www.ncbi.nlm.nih.gov/pubmed/30891813 https://onlinelibrary.wiley.com/doi/10.1111/and.13271
- Simoni M, Brigante G, Rochira V, Santi D, Casarini L. Prospects for FSH Treatment of Male Infertility. J Clin Endocrinol Metab. Jul 1 2020;105(7)doi:10.1210/clinem/dgaa243. https://www.ncbi.nlm.nih.gov/pubmed/32374828
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. Sep 2013;1(5):749-57. doi:10.1111/j.2047-2927.2013.00107.x. https://www.ncbi.nlm.nih.gov/pubmed/23970453 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.2047-2927.2013.00107.x?download=true
- Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Calogero AE, La Vignera S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert opinion on pharmacotherapy. Aug 2019;20(12):1517-1525. doi:10.1080/14656566.2019.1615057.
- Kooshesh L, Bahmanpour S, Zeighami S, Nasr-Esfahani MH. Effect of Letrozole on sperm parameters, chromatin status and ROS level in idiopathic Oligo/Astheno/Teratozoospermia. Reproductive biology and endocrinology : RB&E. May 13 2020;18(1):47. doi:10.1186/s12958-020-00591-2. https://www.ncbi.nlm.nih.gov/pubmed/32404173 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7218838/pdf/12958_2020_Article_591.pdf
- Shuling L, Sie Kuei ML, Saffari SE, et al. Do men with normal testosterone-oestradiol ratios benefit from letrozole for the treatment of male infertility? Reprod Biomed Online. Jan 2019;38(1):39-45. doi:10.1016/j.rbmo.2018.09.016. https://www.ncbi.nlm.nih.gov/pubmed/30449700 https://www.rbmojournal.com/article/S1472-6483(18)30520-0/fulltext
- Visser WR, Smith-Harrison LI, Krzastek SC. Surgical procedures for male infertility: an update. Current opinion in urology. Jan 2021;31(1):43-48. doi:10.1097/MOU.0000000000000828. https://www.ncbi.nlm.nih.gov/pubmed/33165012
- Majzoub A, ElBardisi H, Covarrubias S, et al. Effect of microsurgical varicocelectomy on fertility outcome and treatment plans of patients with severe oligozoospermia: An original report and meta-analysis. Andrologia. Jul 2021;53(6):e14059. doi:10.1111/and.14059. https://www.ncbi.nlm.nih.gov/pubmed/33763931 https://onlinelibrary.wiley.com/doi/10.1111/and.14059
- Gozen AS, Tokas T, Tawfick A, et al. Robot-assisted vasovasostomy and vasoepididymostomy: Current status and review of the literature. Turkish journal of urology. Sep 2020;46(5):329-334. doi:10.5152/tud.2020.20257. https://www.ncbi.nlm.nih.gov/pubmed/32915714 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7483453/pdf/tju-46-5-329.pdf
- Kresch E, Efimenko I, Gonzalez D, Rizk PJ, Ramasamy R. Novel methods to enhance surgical sperm retrieval: a systematic review. Arab J Urol. 2021;19(3):227-237. doi:10.1080/2090598X.2021.1926752. https://www.ncbi.nlm.nih.gov/pubmed/34552774 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8451700/pdf/TAJU_19_1926752.pdf
- Mangum CL, Patel DP, Jafek AR, et al. Towards a better testicular sperm extraction: novel sperm sorting technologies for non-motile sperm extracted by microdissection TESE. Transl Androl Urol. Mar 2020;9(Suppl 2):S206-S214. doi:10.21037/tau.2019.08.36. https://www.ncbi.nlm.nih.gov/pubmed/32257861 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108989/pdf/tau-09-S2-S206.pdf
- Mangoli E, Khalili MA. The Beneficial Role of Intra Cytoplasmic Morphologically Selected Sperm Injection (IMSI) in Assisted Reproduction. J Reprod Infertil. Jan-Mar 2020;21(1):3-10. https://www.ncbi.nlm.nih.gov/pubmed/32175260 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7048693/pdf/JRI-21-3.pdf
- Oseguera-Lopez I, Ruiz-Diaz S, Ramos-Ibeas P, Perez-Cerezales S. Novel Techniques of Sperm Selection for Improving IVF and ICSI Outcomes. Frontiers in cell and developmental biology. 2019;7:298. doi:10.3389/fcell.2019.00298. https://www.ncbi.nlm.nih.gov/pubmed/31850340 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6896825/pdf/fcell-07-00298.pdf
- Pedrosa ML, Furtado MH, Ferreira MCF, Carneiro MM. Sperm selection in IVF: the long and winding road from bench to bedside. JBRA Assist Reprod. Jul 14 2020;24(3):332-339. doi:10.5935/1518-0557.20190081. https://www.ncbi.nlm.nih.gov/pubmed/32155013 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7365522/pdf/jbra-24-03-0332.pdf
- Choe J, Archer JS, Shanks AL. In Vitro Fertilization. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Yang H, Kuhn C, Kolben T, et al. Early Life Oxidative Stress and Long-Lasting Cardiovascular Effects on Offspring Conceived by Assisted Reproductive Technologies: A Review. International journal of molecular sciences. Jul 22 2020;21(15)doi:10.3390/ijms21155175. https://mdpi-res.com/d_attachment/ijms/ijms-21-05175/article_deploy/ijms-21-05175.pdf
- Zhang L, Zhang W, Xu H, Liu K. Birth defects surveillance after assisted reproductive technology in Beijing: a whole of population-based cohort study. BMJ open. Jun 23 2021;11(6):e044385. doi:10.1136/bmjopen-2020-044385. https://www.ncbi.nlm.nih.gov/pubmed/34162637 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8231031/pdf/bmjopen-2020-044385.pdf
- Luke B, Brown MB, Wantman E, et al. The risk of birth defects with conception by ART. Human reproduction (Oxford, England). Jan 1 2021;36(1):116-129. doi:10.1093/humrep/deaa272. https://www.ncbi.nlm.nih.gov/pubmed/33251542 https://watermark.silverchair.com/deaa272.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAskwggLFBgkqhkiG9w0BBwagggK2MIICsgIBADCCAqsGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMR50DanXJ1BTpyJLsAgEQgIICfMczpgqZPO9PlgDwOi9EsN4jhfSyUQ_k3mF1aSSX9PqqyOkc4dWkQlyx50etMyibRTRba8ndFl2MUyiCyDrvfC_gLsFZlZguZuKnxuCvfj8AnrXKwv18vlVxYbcdrCRSq6w6oOmX5D4GNFW961TQZ12U9uY1Jw2oUQSO80mMdnN4pgfX9z_ODhsvgIfTz6RPsQfNr4RgviR8FdNCZsQyC_17YTxZB6xrNXeagyrfDsqrWJFFIYl_MrnQ1a6oz5M3IxOMbhnwH3Bb_wszM59RNTLjIICxEBJ1-ey2-qkg9GlDlJIcAASf6BAho_3sajXwtdoP4-FELNDmsyLJQOYEn4cnC9fIWZHH75bGztNXUnH-709lO7q_jE3a75dlR5Uob9YcjVvTlcFNMdG0I_30l7TsxK1PfdcN5xozkEHghI8lCjw9mD8lsubRVHrf_5_JpBVajtNmuKS6fSEuzkyD9up4GIkJXKnmaMIAoHXaJapID3v6_7bw_6wIIwU2O94HPH_QxF9indU49h-voS-ZEY74GMhElvBt_aPRY1WNvJz4P_nznA-wLHDuCosAMDwq_J85zn1fgmWfN5XzcAG8aVheeagMTFwCG9HP87R53MqUeHnvjuP77O0xcTnZ1lbPUr9yhqtRQ5sW760E1VpEzQFVbTtS48pOoOEI2rgwRd4GuB-XtlM3bNa_UsC-OqFB3RgFAzxOVBSghjHJ96jBTcOrNB-C24z-BuRvm3rm9ivJOXgpXwQzVswdEPu8rdUPzgME-UYgGnw5f2yj3Oh2xtqVIrWhs-0hCnjZY9O_T2q0kx-sh_2GFpX8RNDL_3GbTxqP3lgB2ExM6IxbfA
- Lu YH, Wang N, Jin F. Long-term follow-up of children conceived through assisted reproductive technology. Journal of Zhejiang University Science B. May 2013;14(5):359-71. doi:10.1631/jzus.B1200348. https://www.ncbi.nlm.nih.gov/pubmed/23645173 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3650450/pdf/JZUSB14-0359.pdf
- Yang M, Fan X-B, Wu J-N, Wang J-M. Association of assisted reproductive technology and multiple pregnancies with the risks of birth defects and stillbirth: A retrospective cohort study. Scientific Reports. 2018/05/29 2018;8(1):8296. doi:10.1038/s41598-018-26567-2. https://doi.org/10.1038/s41598-018-26567-2
- Paraskevi L, Antigoni S, Kleanthi G. Stress and Anxiety Levels in Couples who Undergo Fertility Treatment: a Review of Systematic Reviews. Mater Sociomed. Mar 2021;33(1):60-64. doi:10.5455/msm.2021.33.60-64. https://www.ncbi.nlm.nih.gov/pubmed/34012353 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8116083/pdf/MSM-33-60.pdf
- Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ open. Jan 28 2015;5(1):e006592. doi:10.1136/bmjopen-2014-006592. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4316425/pdf/bmjopen-2014-006592.pdf
- Ayeleke RO, Asseler JD, Cohlen BJ, Veltman-Verhulst SM. Intra-uterine insemination for unexplained subfertility. The Cochrane database of systematic reviews. Mar 3 2020;3(3):CD001838. doi:10.1002/14651858.CD001838.pub6. https://www.ncbi.nlm.nih.gov/pubmed/32124980 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059962/pdf/CD001838.pdf
- Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract. Dec 11 2020;6(1):23. doi:10.1186/s40738-020-00092-1. https://www.ncbi.nlm.nih.gov/pubmed/33308319 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7731622/pdf/40738_2020_Article_92.pdf
- Bosch E, Espinos JJ, Fabregues F, et al. ALWAYS ICSI? A SWOT analysis. J Assist Reprod Genet. Sep 2020;37(9):2081-2092. doi:10.1007/s10815-020-01836-0. https://www.ncbi.nlm.nih.gov/pubmed/32578032 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7492350/pdf/10815_2020_Article_1836.pdf
- Haddad M, Stewart J, Xie P, et al. Thoughts on the popularity of ICSI. J Assist Reprod Genet. Jan 2021;38(1):101-123. doi:10.1007/s10815-020-01987-0. https://www.ncbi.nlm.nih.gov/pubmed/33155089 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7823003/pdf/10815_2020_Article_1987.pdf
- Ambar RF, Agarwal A, Majzoub A, et al. The Use of Testicular Sperm for Intracytoplasmic Sperm Injection in Patients with High Sperm DNA Damage: A Systematic Review. World J Mens Health. Jul 2021;39(3):391-398. doi:10.5534/wjmh.200084. https://www.ncbi.nlm.nih.gov/pubmed/32648379 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8255394/pdf/wjmh-39-391.pdf
- Boivin J. How does stress, depression and anxiety affect patients undergoing treatment? Curr Opin Obstet Gynecol. Jun 2019;31(3):195-199. doi:10.1097/GCO.0000000000000539. https://www.ncbi.nlm.nih.gov/pubmed/30893136
- Yamanaka-Altenstein M, Rauch-Anderegg V, Heinrichs N. The link between infertility-related distress and psychological distress in couples awaiting fertility treatment: a dyadic approach. Hum Fertil (Camb). Jul 7 2021:1-15. doi:10.1080/14647273.2021.1948112. https://www.ncbi.nlm.nih.gov/pubmed/34232107 https://www.tandfonline.com/doi/full/10.1080/14647273.2021.1948112
- Iordachescu DA, Gica C, Vladislav EO, et al. Emotional disorders, marital adaptation and the moderating role of social support for couples under treatment for infertility. Ginekologia polska. 2021;92(2):98-104. doi:10.5603/GP.a2020.0173. https://www.ncbi.nlm.nih.gov/pubmed/33448003 https://journals.viamedica.pl/ginekologia_polska/article/download/GP.a2020.0173/52681
- Molgora S, Baldini MP, Tamanza G, Somigliana E, Saita E. Individual and Relational Well-Being at the Start of an ART Treatment: A Focus on Partners' Gender Differences. Frontiers in psychology. 2020;11:2027. doi:10.3389/fpsyg.2020.02027. https://www.ncbi.nlm.nih.gov/pubmed/33117204 https://air.unimi.it/retrieve/handle/2434/800269/1660277/Molgora%20et%20al.%2c%20Front%20Psy%2c%202020.pdf
- O'Connell SBL, Gelgoot EN, Grunberg PH, et al. 'I felt less alone knowing I could contribute to the forum': psychological distress and use of an online infertility peer support forum. Health Psychol Behav Med. Feb 11 2021;9(1):128-148. doi:10.1080/21642850.2021.1884556. https://www.ncbi.nlm.nih.gov/pubmed/34104553 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8158233/pdf/RHPB_9_1884556.pdf
- AN. Adoption Network: Adoption after infertility. Available at https://adoptionnetwork.com/adoptive-parents/how-to-adopt/considering-adoption/adoption-after-infertility/. Accessed 10/18/2021. 2021;
- Rezamahaleh FA, Khadivzadeh T, Asgharinekah SM, Esmaeili H. The effect of face-to-face and telephone counseling on the desire for adoption in infertile couples. J Educ Health Promot. 2021;10(1):164. doi:10.4103/jehp.jehp_964_20. https://www.ncbi.nlm.nih.gov/pubmed/34250098
- Jhuang YH, Chung CH, Wang ID, et al. Association of Obstructive Sleep Apnea With the Risk of Male Infertility in Taiwan. JAMA Netw Open. Jan 4 2021;4(1):e2031846. doi:10.1001/jamanetworkopen.2020.31846. https://www.ncbi.nlm.nih.gov/pubmed/33475753
- Hammoud AO, Carrell DT, Gibson M, Peterson CM, Meikle AW. Updates on the relation of weight excess and reproductive function in men: sleep apnea as a new area of interest. Asian journal of andrology. Jan 2012;14(1):77-81. doi:10.1038/aja.2011.64. https://www.ncbi.nlm.nih.gov/pubmed/22138900
- Lim ZW, Wang ID, Wang P, et al. Obstructive sleep apnea increases risk of female infertility: A 14-year nationwide population-based study. PLoS One. 2021;16(12):e0260842. doi:10.1371/journal.pone.0260842. https://www.ncbi.nlm.nih.gov/pubmed/34910749
- Kyrkou K, Alevrakis E, Baou K, et al. Impaired Human Sexual and Erectile Function Affecting Semen Quality, in Obstructive Sleep Apnea: A Pilot Study. J Pers Med. Jun 16 2022;12(6)doi:10.3390/jpm12060980. https://www.ncbi.nlm.nih.gov/pubmed/35743765
- Peel A, Saini A, Deluao JC, McPherson NO. Sperm DNA damage: The possible link between obesity and male infertility, an update of the current literature. Andrology. Feb 15 2023;doi:10.1111/andr.13409. https://www.ncbi.nlm.nih.gov/pubmed/36789664
- Torres M, Laguna-Barraza R, Dalmases M, et al. Male fertility is reduced by chronic intermittent hypoxia mimicking sleep apnea in mice. Sleep. Nov 1 2014;37(11):1757-65. doi:10.5665/sleep.4166. https://www.ncbi.nlm.nih.gov/pubmed/25364071
- Mannucci A, Argento FR, Fini E, et al. The Impact of Oxidative Stress in Male Infertility. Frontiers in molecular biosciences. 2021;8:799294. doi:10.3389/fmolb.2021.799294. https://www.ncbi.nlm.nih.gov/pubmed/35071326
- Lin PY, Ting H, Lu YT, et al. Risk of Infertility in Males with Obstructive Sleep Apnea: A Nationwide, Population-Based, Nested Case-Control Study. J Pers Med. Jun 5 2022;12(6)doi:10.3390/jpm12060933. https://www.ncbi.nlm.nih.gov/pubmed/35743718
