
Bell's Palsy
Bell's Palsy
Last Section Update: 06/2021
Contributor(s): Maureen Williams, ND; Shayna Sandhaus, PhD
1 Overview
What is Bell’s Palsy?
Bell’s palsy involves dysfunction of a nerve that controls facial muscles, resulting in weakness or paralysis of one side, or more rarely both sides, of the face.
The majority of people with Bell’s palsy begin to recover without treatment within two to three weeks, achieving full recovery within three or four months, but recovery can take longer in some people; permanent loss of function is rare.
What are the Signs and Symptoms of Bell’s Palsy?
- One-sided facial paralysis that occurs over a few hours to a few days. This includes a droop to the mouth on the affected side and an inability to frown, close the eye, or produce tears.
- This can cause the cornea to become dried out which could lead to long-term visual impairment.
- In the large majority of cases, symptoms resolve completely within nine months.
- People who do not have some recovery within 21 days have a greater risk of lasting facial muscle weakness.
It is important to note that symptoms of Bell’s palsy often overlap with those of stroke. For this reason, it is imperative that medical attention be sought as soon as symptoms of facial weakness or paralysis are perceived.
How is Bell’s Palsy Treated?
- A short course of corticosteroids is recommended within three days of symptom onset to reduce inflammation. Corticosteroids have been shown to improve outcomes and recovery at six to 12 months.
- Antiviral therapy is generally only recommended in severe cases.
- Artificial tears and ophthalmic ointments can be used to prevent damage to the cornea.
Top Nutrients for Bell’s Palsy
- Methylcobalamin. Methylcobalamin plus a corticosteroid helped patients recover faster than a corticosteroid alone in a study of subjects who had Bell’s palsy longer than two weeks.
- Acetyl-L-carnitine. A study of subjects with facial palsy found supplementing with acetyl-L-carnitine along with methylprednisolone reduced a measure of paralysis by half, whereas it remained the same with methylprednisolone alone.
- Omega-3 fatty acids. Omega-3 fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are converted into anti-inflammatory compounds that can help protect nerves from damage.
- Niacin. In a series of 74 cases treated with 100‒250 mg of niacin, 73 cases resulted in good facial nerve response within 2‒4 weeks.
- Anti-viral interventions. Natural agents shown to combat viral infection, including licorice, zinc, lysine, and reishi mushrooms, may be of some benefit for those affected by Bell’s palsy.
2 Introduction
“Bell’s palsy” typically refers to acute facial paralysis of unknown origin.1 It results from dysfunction of a nerve that controls the facial muscles, tear glands, and certain salivary gands.1-3 First anatomically identified by Sir Charles Bell in 1821, Bell’s palsy causes weakness or paralysis of one side or, more rarely, both sides of the face.4 Bell’s palsy often manifests rapidly, such that those affected may rise from bed in the morning with inexplicable one-sided facial weakness or paralysis and fear they have experienced a stroke.5
Bell’s palsy is the most common disorder affecting the facial nerve and cause of facial paralysis, with more than 40,000 cases diagnosed each year in the United States.6 Bell’s palsy can occur at almost any age and is equally common in men and women. People with diabetes appear to be at increased risk, and the risk is increased 3-fold during pregnancy, especially in the third trimester and first week postpartum.1,7
The underlying cause of Bell’s palsy is not known in most cases, but inflammation and reactivation of a dormant viral infection affecting the facial nerve, also called the cranial nerve VII, appear to be important contributing factors. Herpes viruses, specifically herpes simplex virus type 1 and herpes zoster virus, which causes chickenpox, are thought to be involved in a substantial proportion of cases.1,2
Most people with Bell’s palsy begin to recover spontaneously within several weeks, achieving full recovery within nine months.8,9 About 70% of the patients completely recover their facial muscle function, even without treatment; however, in about 30% of cases, long-term problems such as facial weakness, involuntary facial movements, or persistent secretion of tears may arise.2
Doctors may use corticosteroids to treat Bell’s palsy as they are helpful in reducing inflammation of the facial nerve and may shorten symptom duration if initiated soon after onset. The rates of complete recovery increase with early use of a corticosteroid.10,11 Antiviral drugs such as valacyclovir (Valtrex) or acyclovir (Zovirax) are sometimes used for the condition. However, some studies suggest antiviral drugs do not provide significant benefits when used alone or together with steroids. Therefore, if there is a benefit with antiviral drugs, the benefit is likely modest at best.12
Given the potential role of inflammation in Bell’s palsy, nutrients that help manage the inflammatory response, such as curcumin and omega-3 fatty acids, may support recovery. In addition, nutrients that support healthy nerve cell function such as vitamin B12, acetyl-L-carnitine, and ginkgo may benefit those with Bell’s palsy. Nutrients that inhibit herpes viruses, such as reishi mushroom, licorice, zinc, and L-lysine, may also be supportive.6,13-30
In this protocol, you will learn about the biology of Bell’s palsy and its possible triggers. You will also learn how to recognize the signs and symptoms of this condition, and how it is diagnosed and treated. Finally, you will learn about novel and emerging therapies as well as nutrients that may help speed recovery and reduce symptom severity.
3 Causes
Bell’s palsy has historically been considered an idiopathic condition (ie, a condition that arises spontaneously without a known cause). There are several theories regarding underlying mechanisms.
The most consistent underlying feature of Bell’s palsy is acute inflammation or edema of the facial nerve.8,12,31 The facial nerve passes through small canals in the skull along its path to the facial muscles. When a dormant virus (generally assumed to be a herpes simplex virus) is reactivated and migrates to the facial nerve, an inflammatory response ensues leading to swelling and compression of the nerve within these small bony passages. Non-infectious factors, such as autoimmune processes, may also cause inflammation of the facial nerve and contribute to Bell’s palsy.2,32-35
Inflammation and anatomical factors can result in blood vessel compression and ischemia (decreased blood flow and poor oxygenation) of the facial nerve. Ischemia, which can cause thickening of connective tissue and further nerve compression, has been proposed to be a factor in cases of Bell’s palsy that do not resolve.8,36
It is widely thought that reactivation of latent viral infection triggers the onset of Bell’s palsy in many cases.6 Herpes simplex virus-type 1 (HSV-1) is the most studied with regard to its relationship with Bell’s palsy.8 HSV-1 and other herpes viruses generally manifest in two distinct stages: during the first stage, there is active symptomatic infection and viral replication, whereas during the second stage, the virus is dormant (or latent), residing in the nervous system.37,38 The dormant virus may become reactivated and give rise to another active infection.39 Although some evidence suggests Bell’s palsy may be a manifestation of a reactivated herpes virus, such as HSV-1 or herpes zoster virus (also called varicella zoster virus, the cause of chickenpox and shingles), studies have not been able to consistently link the presence of either of these viruses with a majority of Bell’s palsy patients.40,41 Moreover, Bell’s palsy is not typically associated with other classic signs and symptoms of herpes viruses such as lesions, itching, and pain.
Borrelia burgdorferi , the bacteria that causes Lyme disease, is an important possible cause of facial nerve palsy.42 Other infectious viruses have also been suggested as triggers, including adenovirus, Coxsackievirus, cytomegalovirus, Epstein-Barr virus, mumps, and rubella.43
Another possible underlying mechanism involves an autoimmune process in which immune cells become activated against the myelin proteins specific to the facial nerve. This type of immune reaction may be triggered by an infection or, in rare cases, certain vaccines.9,47-49
4 Risk Factors and Associated Conditions
Bell’s palsy can occur at any age, but it most commonly affects people between ages 15 and 45.6 Although Bell’s palsy appears to affect men and women at similar rates, young women were found to be at greater risk of recurrent Bell’s palsy in one small study.63 Evidence suggests Bell’s palsy may run in some families, and at least one genetic variant has been identified as a possible risk factor.1,64
Several other conditions have been reported to have associations with Bell’s palsy, although the mechanisms behind these relationships is unclear.
- Infection. Recent viral infections, such as due to herpes viruses or respiratory viruses, have been linked to Bell’s palsy risk.5,65-67 In addition, Mycoplasma pneumoniae has been found more frequently in those diagnosed with Bell’s palsy.68 Bell’s palsy can also be a symptom of Lyme disease.69 In one study, 14.7% of Bell’s palsy patients without a known cause had confirmed infection with the bacteria that causes Lyme disease.60
- Pregnancy. The risk of Bell’s palsy increases 2- to 4-fold during late pregnancy and the postpartum period, and increased nerve compression is thought to be a contributing factor.70-72 Bell’s palsy has been noted to be more common in women with other pregnancy complications, such as high blood pressure and preeclampsia, and may be an indicator of further pregnancy-related problems.73,74 Women who develop Bell’s palsy during or just after pregnancy are more likely to have severe symptoms, less likely to receive timely treatment with corticosteroids, and have poorer outcomes than non-pregnant Bell’s palsy patients.70,75
- Stroke. One study found individuals who experienced Bell’s palsy had a 2-fold increase in stroke risk during 2.9 years of monitoring compared with those who had not had Bell’s palsy. Treatment of Bell’s palsy with corticosteroids was found to mitigate the increase in stroke risk somewhat, but those who received corticosteroids still had a 67% increased stroke risk.76
- Anxiety. Anxiety and Bell’s palsy share certain risk factors and may be interrelated. In one study, anxiety patients were found to have a 53% increased risk of developing Bell’s palsy, and Bell’s palsy patients were found to have a 59% increased risk of developing an anxiety disorder.77
- Diabetes. Diabetes increases the risk of developing Bell’s palsy and is present in 5‒10% of people with Bell’s palsy.1,2 In addition, the presence of diabetes has been correlated with a decreased rate of recovery from Bell’s palsy.78,79
- Hypertension. High blood pressure has been associated with an increased risk of, and poorer recovery from, Bell’s palsy.79,80 Individuals whose high blood pressure was well controlled were found in one study to have a better prognosis than those with uncontrolled hypertension.78
- Obesity. Obesity has been linked to an increased risk of Bell’s palsy and has been associated with a poorer recovery rate.81,82
- Metabolic syndrome. Hypertension, abdominal obesity, and high blood sugar are hallmarks of metabolic syndrome and are associated with increased risk of Bell’s palsy. Two other components of metabolic syndrome, high triglyceride and low high-density lipoprotein (HDL) cholesterol levels, have also been correlated with greater odds of developing Bell’s palsy, and Bell’s palsy patients with metabolic syndrome were found to have a lower rate of recovery.79
- Migraine. Migraine sufferers in general have a slightly increased risk of Bell’s palsy. However, among adults between 30 and 60 years old, migraine patients were found to have a 28% increased risk of Bell’s palsy.83
- Cancer. In one study, the risk of being diagnosed with cancer, especially oral cancer, was 43% higher during the five years following a Bell’s palsy diagnosis.84
5 Signs and Symptoms
The classic sign of Bell’s palsy is a facial paralysis that typically occurs on one side and comes on over a matter of hours to a few days (Figure 1).1,45 The paralysis is either partial (one-third of cases) or total (two-thirds of cases) and affects both the upper and lower facial muscles.45,85 Sometimes both sides of the face are affected simultaneously, but this is rare; one study found that bilateral simultaneous facial palsy occurred in less than 1% of cases.86
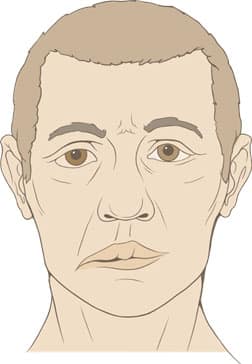
of one-sided facial paralysis
in Bell’s palsy.87
Typically, the facial weakness worsens during the first few days and improves thereafter.85,88 The forehead will stop furrowing and the corner of the mouth will droop on the affected side. Another feature of Bell’s palsy is an inability to close the eye as a result of the lower eyelid drooping. Attempting to close the eye can cause the eyeball to roll upward, known as Bell’s phenomenon.89 Frowning and pursing of the lips are also impaired by Bell’s palsy.
Some individuals with Bell’s palsy describe a feeling of numbness in their face, although facial sensation is generally unchanged.85 Bell’s palsy can impair the formation of tears (lacrimation), resulting in dryness of the eye. However, because Bell’s palsy also impairs control of the eyelids, tears may spill out of the affected eye giving the appearance of over-tearing.85,89 In some cases, parts of the facial nerve that supply the tongue and ear can be affected resulting in loss of taste on the front two-thirds of the tongue and intensification of loud noises.85 Bell’s palsy may produce psychologically troubling symptoms such as drooling, difficulty eating and drinking, facial distortion, and inability to smile.6,9
In most cases, symptoms of Bell’s palsy resolve completely. However, in about 30% of cases, the facial nerve does not recover completely resulting in permanent facial muscle weakness or partial paralysis.2 Another long-term complication, known as synkinesis, occurs when regenerating nerve fibers form disorganized or misdirected connections. This can cause some facial muscles to involuntarily contract along with other muscles, or tear ducts to become active during eating.2 In addition, eye dryness and an inability to close the eyes completely can cause the cornea to become scratched or ulcerated, resulting in lasting vision problems.2,9 Generally speaking, the more severe the muscle weakness or paralysis during the acute stage, the greater the likelihood for long-term muscle weakness. People who do not have some recovery within 21 days or worsen after that time should be reevaluated for other potential etiologies of facial paralysis.12
Consulting a physician as soon as possible after symptom onset is important because delayed diagnosis and treatment may increase the likelihood of long-term problems in some cases. Special consideration must be given in cases involving changes to hearing and the inability to close an eye, which increases the risk of corneal abrasion and ulceration.9
6 Diagnosis
Bell’s palsy is a clinical diagnosis based on symptom history and physical exam findings.85 The physician may ask the patient to “show me your teeth” (for palsy of lower facial muscles) and “close your eyes” (for assessing upper facial muscles). The timing of symptom onset is also important as Bell’s palsy typically has a sudden onset and progression as opposed to other causes of facial palsy such as tumors, which typically cause a gradual progression of muscle weakness over the course of weeks.5
A medical history that includes current or recent pregnancy, diabetes, and recent infection supports a Bell’s palsy diagnosis.5 Multiple family members with a history of Bell’s palsy also point to this diagnosis. Notably, certain familial cases of Bell’s palsy may be associated with increased risk of lasting problems with control of the eyelid, dry eye, and crying when eating.93
Examination of the ear, mouth, head, neck, and skin is important for ruling out other possible causes of facial paralysis.88 Notably, although Bell’s palsy is rare in children younger than 10 years old, up to 50% of reported cases of facial paralysis in this population are attributable to Lyme disease.1,2
Additional Testing
Additional testing is not typically needed for Bell’s palsy. However, laboratory tests such as a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Lyme titer, varicella zoster antibody test, electrolytes, blood urea nitrogen (BUN), creatinine, and liver function tests may help if another cause of facial weakness is likely. Cerebrospinal fluid tests are sometimes recommended to conclusively identify infectious causes such as Lyme disease, shingles, or other viral infections.9 Imaging studies such as magnetic resonance imaging (MRI) may also help rule out a tumor in the case of a gradual onset of facial weakness or paralysis.85
Electroneurography
Electroneurography can help provide prognostic information for people with complete facial muscle paralysis or those who have atypical symptoms or no improvement after several months. Electroneurography uses electricity to stimulate the facial muscle on both sides of the face. The response of the facial muscle evoked by the stimulus is lessened on the side of the face affected by Bell’s palsy, and the degree of nerve degeneration can be quantified by comparing the responses on both sides of the face.1,94
Grading Bell’s Palsy Severity
The severity of Bell’s palsy can be graded using different scales. Two commonly used systems are the House-Brackmann Facial Nerve Grading System and Sunnybrook Facial Grading System.95 The House-Brackmann system allows clinicians to classify facial nerve problems into six different categories based on facial muscle strength and function and appearance of the face at rest. The system also allows clinicians to evaluate progression and recovery and monitor response to treatment.96,97 The Sunnybrook scale measures facial symmetry at rest and during voluntary and involuntary movements and scores them on a scale of 0 to 100. Although both systems can be used to help clinicians assess the severity of Bell’s palsy, they may not adequately assess aspects of Bell’s palsy such as facial comfort and problems with tearing that can affect quality of life.95
7 Treatment
Corticosteroids
Treatment for Bell’s palsy hinges on early intervention with corticosteroids, which are used to reduce inflammation. Multiple randomized controlled trials and meta-analyses confirm corticosteroid therapy, if initiated within 72 hours of symptom onset, increases the likelihood and speed of recovery of facial muscle function.9 A 10-day course of corticosteroids (ie, 10 days of 50 mg of prednisolone or 5 days of 60 mg prednisolone followed by a 5-day taper) is generally recommended.5,9
Antiviral Medications
Antiviral medications, such as acyclovir and valacyclovir, are sometimes added to the corticosteroid regimen in severe cases, but studies have been inconclusive as to whether antivirals offer any significant benefit.5,12,98
Eye Health Maintenance
The impaired tearing that accompanies Bell’s palsy can put the eye at risk for damage. The cornea can become dry and scratched, which can lead to permanent vision problems. It is recommended that, while awake, patients use artificial tears four times daily or every hour if needed and apply ophthalmic ointments at night. Eye patches or protective glasses may also help.12 Targeted exercises may help in cases with residual muscle weakness or dysfunction, and specialized eyelid surgeries, such as customized weighted implants and horizontal lid tightening, may be indicated in cases of persistent inability to fully close the eyelid.9
8 Novel and Emerging Treatments
Low-Level Laser Therapy
Low-level laser therapy, sometimes known as cold laser or photobiomodulation therapy, utilizes wavelengths of light between 660 nm and 905 nm. Whereas high-level laser therapy generates heat and can be used to cut or destroy tissue such as growths or tumors, low-level laser therapy stimulates tissue repair, reduces inflammation, and relieves pain without generating heat.99 In Bell’s palsy, low-level laser therapy is believed to enhance healing and regeneration of nerves by increasing blood flow to affected tissues and decreasing inflammation.100
In a randomized controlled trial in 48 subjects being treated for Bell’s palsy with facial massage and exercise, the addition of low-level laser therapy was more effective than facial massage and exercise alone.101 Another randomized controlled trial in 46 Bell’s palsy patients found low-level laser therapy, in conjunction with facial exercise, led to greater improvement after both three and six weeks than facial exercise alone.102 In an uncontrolled study, 11 of 14 Bell’s palsy patients experienced complete or significant recovery after 12 sessions of low-level laser therapy.103 Another uncontrolled trial found low-level laser therapy resulted in complete recovery in 18 and partial recovery in six of 30 Bell’s palsy patients with poorly controlled type 2 diabetes who were not being treated with corticosteroids as this therapy can further worsen blood sugar regulation.104
In a case report, a 3-year-old child with Bell’s palsy recovered facial muscle function after three weeks of treatment with low-level laser therapy consisting of 11 sessions lasting 15‒20 minutes each.105 A positive response to three sessions of low-level laser therapy was reported in the case of a 13-year-old child with Bell’s palsy; and in a 71-year-old Bell’s palsy patient, recovery was remarkable after five sessions and complete after 10 sessions of low-level laser therapy.106,107
Despite these promising findings, not all studies have noted efficacy, and more research is needed to identify the optimal intensity and duration of laser therapy.108
Facial Fillers and Botulinum Injections
Two potential long-term effects of Bell’s palsy are facial asymmetry and a condition called synkinesis, in which a voluntary facial movement results in the involuntary movement of a different facial muscle group.109,110 A case study examined the effects of injections of facial fillers (eg, hyaluronic acid gel fillers) and botulinum toxin (Botox) injections for the treatment of a 50-year-old woman with these complications from Bell’s palsy. Multiple rounds of these treatments improved facial asymmetry and muscle spasms, reducing pain and improving her quality of life.111 Botulinum injections can also be administered to the non-affected side of the face to improve facial symmetry and cosmetic appearance in people with Bell’s palsy.112
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy involves sitting in a pressurized (hyperbaric) chamber breathing 100% oxygen for approximately an hour. These treatments increase the amount of oxygen in the blood and may help increase oxygen delivery to the facial nerve, and thus have been suggested to help speed recovery after Bell’s palsy.113
Although there is a scarcity of well-designed randomized controlled trials examining the effects of hyperbaric oxygen on Bell’s palsy, one study did compare the effects of hyperbaric oxygen treatments to corticosteroids for people with Bell’s palsy.113,114 This study used one-hour sessions in the hyperbaric oxygen chamber, twice daily, five days per week for up to three weeks or until recovery along with oral placebo. This treatment regimen was compared with normal atmospheric oxygen (21%) with 450 mg of prednisone delivered over eight days. After nine months greater than 95% of people receiving hyperbaric oxygen therapy had fully recovered compared with less than 76% of those receiving prednisone. Furthermore, the hyperbaric oxygen-treated group showed significant improvement in nerve excitability measures compared with the prednisone-treated group.114 On the basis of these preliminary findings, hyperbaric oxygen therapy may represent a potential treatment modality for Bell’s palsy, especially since it has minimal side effects.
Infusion Therapy
Infusion therapy is an approach for treating Bell’s palsy using corticosteroids mixed in an infusion solution containing dextran and pentoxifylline. The corticosteroids reduce inflammation and the combination of dextran and pentoxifylline increases blood flow to the nerve.115 Although this approach has not been heavily researched in recent years, multiple older studies in Europe and Asia have found it to be an effective method of treating Bell’s palsy and suggest it may be more effective than steroids alone.115-118
One study compared the effectiveness of seven days of infusion therapy with 500 mL dextran solution plus 500 mg hydrocortisone (starting at 500 mg and tapering down to 100 mg) against an orally administrated steroid. Patients with complete paralysis who received infusion therapy had a significantly better recovery rate compared with the oral steroid group.115 Another study by the same investigators modified the infusion therapy by adding pentoxifylline. It was found that 87% of patients with complete palsy had total recovery when treated with the modified infusion therapy compared with 68% of those receiving oral steroids in combination with vasodilators, adenosine triphosphate, and vitamins.116 Finally, a retrospective study of 239 people with Bell’s palsy found that a dextran and pentoxifylline infusion combined with prednisone (starting dose 250 mg and tapering over 18 days) provided total recovery in 92% of people with complete facial paralysis and 98% of people with partial facial paralysis; results were superior when therapy started within three days of developing palsy.117,118
Nerve Growth Factor
Nerve growth factor is a protein with neurotrophic effects. Like all neurotrophic factors, it is involved in regulating the growth, maintenance, and survival of neurons. Nerve growth factor was the first neurotrophic factor to be discovered and is widely used in China to promote nerve repair and regeneration in a range of neurological conditions, including Bell’s palsy. A meta-analysis of eight clinical trials performed in China found the inclusion of nerve growth factor in Bell’s palsy treatment was likely to improve outcomes. In the clinical trials, nerve growth factor was administered via intramuscular injections in doses of 30 mcg daily for seven days or 4 mcg daily for 7–28 days. Although the analysis of findings suggested favorable effects for nerve growth factor, the researchers were unable to draw a firm conclusion due to low quality and quantity of evidence.119
9 Dietary and Lifestyle Interventions
Low-Arginine/High-Lysine Diet
Since Bell’s palsy may be linked to reactivation of the herpes simplex virus, dietary strategies that inhibit this virus may also prove effective for treating or shortening the duration of Bell’s palsy. Arginine is an amino acid that appears to be particularly important for the replication of herpes simplex virus. Multiple studies have found that depriving herpes simplex virus of arginine can halt its replication.120,121 On the other hand, the amino acid lysine impairs the virus’ ability to use arginine, thereby slowing viral replication. Avoiding foods such as peanuts, tree nuts, and chocolate, which are high in arginine and low in lysine, may help suppress the herpes virus.13
Acupuncture
Multiple studies have found that acupuncture can help improve symptoms and return normal facial function in people with Bell’s palsy. A meta-analysis that included 11 randomized controlled trials with a combined total of 1,258 participants found acupuncture increased the likelihood of cure and may have been more effective than medications for treating Bell’s palsy.122 A previous meta-analysis of 14 trials with 1,541 participants similarly found acupuncture may enhance recovery in Bell’s palsy patients.123 However, methodological differences in the clinical trials have made it impossible to say with certainty whether acupuncture is an effective therapy for this condition. MRI studies show Bell’s palsy is associated with abnormal functional networks in the brain that are normalized through successful acupuncture treatment.124,125 Electroacupuncture, a technique in which a low level of electrical current is passed between two acupuncture needles, may lead to improved outcomes over typical manual acupuncture.126 Factors such as being younger, normal weight, being in the acute stage of the condition, and with less severe symptoms have been shown to increase the likelihood of benefiting from acupuncture.127
Biofeedback
Biofeedback can help people with incomplete recovery from Bell’s palsy learn to control facial muscles by responding to reinforcement cues. Biofeedback strategies that use mirrors and measurements of muscle activation (electromyography or EMG) to reinforce muscle control have been shown to improve facial symmetry and reduce abnormal muscle movements in patients with long-term consequences from Bell’s palsy.128-130 One study reported on the effect of mirror biofeedback training combined with botulinum toxin injection therapy in 27 cases of facial synkinesis following facial palsy. Improvement was noted at each of four evaluations, and lasting improvement was attributed to the biofeedback portion of treatment program.131 Another study with 22 participants found mirror biofeedback enhanced the effectiveness of standard physical rehabilitation.132 Other biofeedback techniques involving taping of facial regions to build sensory awareness have also been reported to improve facial muscle control.133,134
Physical Therapy
Physical therapy may also help people with Bell’s palsy recover muscle function. While facial exercises (eg, practicing emotional expressions) may help improve muscle function and reduce involuntary facial movements in people with Bell’s palsy,135 the overall evidence of benefit is sparce.136 Physical rehabilitation, consisting of stretching and manual and verbal input, applied at an early stage resulted in overall improvement of the condition of Bell’s palsy patients and accelerated their recovery compared with non-rehabilitated patients.137 The overall usefulness of physical rehabilitation therapies in Bell’s palsy is still uncertain.138
10 Nutrients
Nutrients that promote neuronal health, quell inflammation, and inhibit viral activity may confer benefits by targeting mechanisms underlying Bell’s palsy. Other nutrient interventions that may be beneficial are described in the Inflammation and Herpes and Shingles protocols.
Vitamin B12
Vitamin B12 is important for neuronal health.139,140 In a controlled trial comparing three treatment regimens in 60 subjects with Bell’s palsy of greater than two weeks duration, the first group received 500 mcg intramuscular injections of methylcobalamin (the active form of vitamin B12) three times weekly for at least eight weeks or until recovery, the second received 60 mg prednisolone daily tapered over three weeks, and the third received methylcobalamin plus prednisolone. After one week of treatment, the methylcobalamin and methylcobalamin plus prednisolone groups improved significantly, but the prednisolone alone group showed only slight improvement. It took, on average, about two weeks for participants in the methylcobalamin and methylcobalamin plus prednisolone groups to achieve complete recovery versus nine and a half weeks in the prednisolone only group.14
A meta-analysis that included five randomized controlled trials with a total of 344 Bell’s palsy patients found vitamin B12 injections along with acupuncture reduced the likelihood of incomplete recovery compared with acupuncture alone.141 Because vitamin B12 is generally safe and relatively inexpensive, it represents an intriguing natural option for treating Bell’s palsy.142
Acetyl-L-Carnitine
Acetyl-L-carnitine, a compound found throughout both the central and peripheral nervous systems, plays a role in several processes, including the processing of fatty acids. A randomized placebo-controlled trial in 43 subjects with idiopathic facial palsy found those given 3 grams of oral acetyl-L-carnitine along with 50 mg methylprednisolone daily for 14 days had more rapid functional recovery of the facial nerve compared with those given methylprednisolone plus placebo. After 10 days of treatment, a measure of paralysis was reduced by half in the acetyl-L-carnitine group but unchanged in the placebo group.15 Additionally supporting the use of acetyl-L-carnitine are studies with the nutrient in other conditions involving nerve damage. Preclinical research showed acetyl-L-carnitine prevented and mitigated nerve pain caused by the chemotherapy drug paclitaxel (Abraxane).143 Two clinical studies found acetyl-L-carnitine to be effective for treating various forms of neuropathy, including diabetic and drug-induced neuropathies.144
Additional Support
Given the relatively benign nature of Bell’s palsy, its typically limited disease course, and good prognosis in most cases, few human clinical trials have examined nutritional interventions. Nevertheless, owing to the inflammation and nerve involvement in Bell’s palsy, several nutrients that promote neuronal health and mitigate inflammation may be helpful.
Niacin. Niacin (vitamin B3) is a vitamin that can cause blood vessels to dilate, which is why it causes flushing in many people. This increased blood flow has been proposed to help speed the healing of the facial nerve. Some evidence suggests niacin, administered orally or via intramuscular injection, may be useful in treating Bell’s palsy, although data are very limited. In an older series of 74 cases treated with 100‒250 mg niacin, all but one case resulted in good facial nerve response within 2‒4 weeks, such that an observer would be unable to point out which side of the face was paralyzed. In 39 of the 74 cases, treatment began within two days of the onset of paralysis and these individuals had complete recovery within 14 days.145
Omega-3 fatty acids. Omega-3 fatty acids can reduce inflammation in the body. The omega-3 fatty acids commonly obtained from fish, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), inhibit signaling of a pro-inflammatory molecule called nuclear factor-kappa B (NF-ĸB) and reduce levels of other chemicals that trigger inflammation, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6.146 These fatty acids are also metabolized into compounds called resolvins that protect nerves from inflammatory damage.147 Some preclinical evidence suggests omega-3 fatty acids may help improve recovery after nerve damage while in healthy people they have been shown to improve motor nerve function.148 In type 1 diabetics, fish oil supplementation has also been shown to significantly improve nerve growth.150 While no studies have been carried out in the context of Bell’s palsy, it is reasonable to suggest omega-3 fatty acids may offer some support for inflammatory nerve damage and recovery of function.
Ginkgo. Numerous studies indicate ginkgo (Ginkgo biloba) leaf extract has neuroprotective effects,151 which may make it a helpful therapeutic for Bell’s palsy. For example, ginkgo has been found to inhibit free radical damage to nerve cell membranes, reduce neuroinflammation, improve neuronal mitochondrial function and energy production, and promote nerve growth and formation of neuronal connections.152-155 In preclinical studies, ginkgo extracts have been found to enhance nerve healing after injury related to trauma, toxin exposure, ischemia (lack of oxygen), and oxidative stress.156-160 An active component from ginkgo was found to promote regeneration of myelin in part by reducing inflammatory signaling in animal and cellular models of a demyelinating disorder.153 A compound from ginkgo has also demonstrated antiviral activity against herpes viruses in the laboratory.161
Curcumin. Curcumin, a carotenoid found in turmeric that has potent anti-inflammatory properties, has been shown to help protect against nerve damage caused by diabetes and alcohol, possibly by protecting neurons from inflammation-induced damage.18,19 Specifically, curcumin is able to reduce inflammation by increasing the activity of proteins that inhibit the activity of NF-ĸB, a major trigger for inflammation. Curcumin has been shown to protect neurons from damage in preclinical models of stroke, Alzheimer disease, and oxidative stress.20,162-164
Green tea extract. Green tea contains high amounts of polyphenols known as catechins, including epigallocatechin gallate (EGCG), that scavenge free radicals, inhibit oxidative injury, and lower oxidative stress levels. Preclinical research indicates EGCG may specifically protect neurons by suppressing inflammatory signaling by activated immune cells in the nervous system and promoting release of an important nerve growth factor called brain-derived neurotrophic factor (BDNF).165,166 Animal research suggests EGCG may also reduce body-wide and nervous system inflammation related to metabolic disorders, which increase the risk of Bell’s palsy.167,168 Animal models show EGCG may play a role in preventing a range of neurological disorders.165,169,170
Vitamin E. Vitamin E, comprising tocopherols and tocotrienols, has free radical-scavenging and anti-inflammatory effects. It has particular neuroprotective effects because of its nature as a lipid compound, and has been suggested to be beneficial in a range of neurological disorders.171-173 Studies in animals have shown vitamin E has the potential to protect against oxidative and inflammation-related nerve damage.173-175 Other animal research indicates adequate vitamin E levels are needed for an effective immune response to HSV infection.176
Alpha-lipoic acid. Alpha-lipoic acid, a free radical-scavenging nutrient involved in glucose and fat metabolism,177,178 is used to treat metabolic disorders like obesity and diabetes, as well as neurological complications such as diabetic neuropathy, dry eyes, and retinopathy.179-181 Alpha-lipoic acid has been shown to improve nerve function in animal and laboratory models of neurological dysfunction, as well as in some clinical research.178
Licorice. Licorice (Glycyrrhiza glabra) has been used traditionally to combat viral infections. It contains compounds that exert strong anti-inflammatory effects and have demonstrated antiviral activity against several viruses, including HSV-1 and varicella zoster virus, in preclinical studies.21,182,183
Zinc. Zinc is an important nutrient for normal nerve function, and deficiency can cause hearing and taste disturbances and contribute to depression and neurological disorders.184 Zinc supports nerve growth and the formation of new neuronal connections.185 It also inhibits the replication of HSV-1 and has been effectively used in multiple clinical trials to reduce the duration of herpes outbreaks.13,186
Lysine. Lysine is an amino acid sometimes used to treat HSV infections.22 A double-blind placebo-controlled trial that included participants with oral-facial or genital herpes found consuming 1 gram L-lysine three times daily for six months reduced the frequency, duration, and severity of herpes outbreaks.23 Findings from other studies suggest lysine supplementation, particularly at doses higher than 3 grams per day, may reduce frequency, shorten duration, and decrease severity of cold sores due to HSV, but the quality of supportive evidence is low.29,187 Although lysine has not been evaluated in the context of Bell’s palsy, its possible anti-herpes activity suggests it may be helpful.
Reishi mushroom. Reishi mushroom (Ganoderma lucidum) has been used medicinally for centuries in China, Japan, and Korea.188 Researchers have identified two different compounds in the reishi mushroom, known as GLPG (Ganoderma lucidum proteoglycan) and APBP (acidic protein bound polysaccharide), that showed strong antiviral activities against both HSV-1 and HSV-2 in the laboratory setting.189-191 Reishi compounds have also been shown to reduce inflammation, lower oxidative stress, and strengthen immune defenses.192
Herbal preparations containing reishi mushroom have shown promising results in uncontrolled clinical trials in subjects with herpes virus outbreaks. A combination of six medicinal herbs, including reishi, was reported to reduce pain caused by shingles in five cases. Other trials reported the same reishi-containing herbal mixture reduced pain and shortened symptom duration in patients with recurrent oral and genital herpes outbreaks.193-195
Lemon balm. Lemon balm (Melissa officinalis) is a plant in the mint family used traditionally to treat herpes outbreaks and other viral infections.196 Multiple laboratory experiments have shown lemon balm extracts possess antiviral activities against both HSV-1 and HSV-2.197-203 Clinical trials have evaluated the efficacy of topical lemon balm preparations and shown positive results. In a double-blind placebo-controlled trial with 66 participants, a lemon balm ointment, applied four times daily for five days, improved symptoms of oral herpes, shortened healing time, and prevented spread of the infection. The authors further suggested lemon balm may increase time between outbreaks.204 Two additional trials involving 115 and 116 patients found topical therapy with lemon balm extract eased oral herpes symptoms and was most effective when used early.205 Lemon balm has also been noted to protect nerves from injury due to inflammation, hypoxia (low oxygen level, such as due to poor circulation), and oxidative stress in laboratory and animal models.206-209
Propolis. Propolis, a resin-like substance obtained from beehives, has a long history of medicinal use.210 It contains a mixture of several compounds, including flavonoids and other polyphenols, that have strong anti-inflammatory and oxidative stress-reducing effects.211 Some propolis-derived compounds have demonstrated antimicrobial activity, including antiviral activity against HSV-1 and HSV-2.211-213 Comprehensive reviews have suggested propolis may be beneficial in treating herpes virus-related skin lesions, but available research is not high quality.214,215 In clinical trials in subjects with herpes lesions on the lips, a 0.5% topical propolis extract solution was more effective than topical use of the antiviral drug acyclovir for shortening time to healing and reducing symptoms such as pain, burning, itching, tension, and swelling.216,217 With its combination of antiviral and anti-inflammatory properties, propolis has the potential to be beneficial in Bell’s palsy.
Fucoidans. Fucoidans are naturally occurring polysaccharides found in edible brown algae that have been found to promote balanced immune function in preclinical models.218 They also strengthen the antiviral immune response and demonstrate direct antiviral actions, including against HSV-1 and HSV-2, in preclinical research.219-221 Additionally, fucoidans have the capacity to reduce inflammation and oxidative stress.218 A report on two case studies noted use of a topical 4% fucoidan cream markedly decreased healing time associated with recurrent oral herpes outbreaks.222
Other anti-inflammatory and antiviral nutrients. Several other nutrients with anti-inflammatory, antiviral, and neuroprotective properties may play a role in speeding healing and recovery in patients with Bell’s palsy. These include vitamins A, C, and D.223-228 Flavonoids such as resveratrol and quercetin, as well as flavonoid-rich elderberry extract, also have these properties and have the potential to help promote Bell’s palsy recovery.228-230 Probiotics may modulate immune function and support neuronal health and regeneration.231
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Ronthal M, Greenstein P. Bell's palsy: Pathogenesis, clinical features, and diagnosis in adults. UpToDate. Updated 5/11/2020. Accessed 4/12/2021, https://www.uptodate.com/contents/bells-palsy-pathogenesis-clinical-features-and-diagnosis-in-adults?search=Bell's%20Palsy&source=search_result&selectedTitle=2~73&usage_type=default&display_rank=2
- Zandian A, Osiro S, Hudson R, et al. The neurologist's dilemma: a comprehensive clinical review of Bell's palsy, with emphasis on current management trends. Med Sci Monit. Jan 20 2014;20:83-90. doi:10.12659/msm.889876
- Kosins AM, Hurvitz KA, Evans GR, Wirth GA. Facial paralysis for the plastic surgeon. Can J Plast Surg. Summer 2007;15(2):77-82. doi:10.1177/229255030701500203
- Sajadi MM, Sajadi MR, Tabatabaie SM. The history of facial palsy and spasm: Hippocrates to Razi. Neurology. Jul 12 2011;77(2):174-8. doi:10.1212/WNL.0b013e3182242d23
- Baugh RF, Basura GJ, Ishii LE, et al. Clinical practice guideline: Bell's palsy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery . Nov 2013;149(3 Suppl):S1-27. doi:10.1177/0194599813505967
- NINDS. National Institue of Neurological Disorders and Stroke. Bell’s Palsy Fact Sheet. Available at https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Bells-Palsy-Fact-Sheet#3050_2 . Last updated 10/02/2020. Accessed 03/13/2021. . 2020;
- Riga M, Kefalidis G, Danielides V. The role of diabetes mellitus in the clinical presentation and prognosis of Bell palsy. J Am Board Fam Med. Nov-Dec 2012;25(6):819-26. doi:10.3122/jabfm.2012.06.120084
- Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell's palsy: a review. J Neurol. Jul 2020;267(7):1896-1905. doi:10.1007/s00415-019-09282-4
- Heckmann JG, Urban PP, Pitz S, Guntinas-Lichius O, Gágyor I. The Diagnosis and Treatment of Idiopathic Facial Paresis (Bell's Palsy). Deutsches Arzteblatt international. Oct 11 2019;116(41):692-702. doi:10.3238/arztebl.2019.0692
- Prud’hon S, Kubis N. La paralysie faciale périphérique a frigore. La Revue de Médecine Interne. 2019/01/01/ 2019;40(1):28-37. doi:https://doi.org/10.1016/j.revmed.2018.03.011
- Somasundara D, Sullivan F. Management of Bell's palsy. Aust Prescr . Jun 2017;40(3):94-97. doi:10.18773/austprescr.2017.030
- Ronthal M, Greenstein P. Bell's palsy: Treatment and prognosis in adults. UpToDate. Updated 2/2/2021. Accessed 4/21/2021, https://www.uptodate.com/contents/bells-palsy-treatment-and-prognosis-in-adults?search=Bell's%20Palsy&source=search_result&selectedTitle=1~73&usage_type=default&display_rank=1
- Gaby AR. Natural remedies for Herpes simplex. Altern Med Rev. Jun 2006;11(2):93-101.
- Jalaludin MA. Methylcobalamin treatment of Bell's palsy. Methods and findings in experimental and clinical pharmacology. Oct 1995;17(8):539-44.
- Mezzina C, De Grandis D, Calvani M, Marchionni A, Pomes A. Idiopathic facial paralysis: new therapeutic prospects with acetyl-L-carnitine. International journal of clinical pharmacology research. 1992;12(5-6):299-304.
- Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive care medicine. Sep 2008;34(9):1580-92. doi:10.1007/s00134-008-1142-4
- Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. Oct 2010;35(11):2238-48. doi:10.1038/npp.2010.98
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. Mar 5 2012;511(1):18-22. doi:10.1016/j.neulet.2012.01.019
- Kulkarni SK, Dhir A. An overview of curcumin in neurological disorders. Indian journal of pharmaceutical sciences. Mar 2010;72(2):149-54. doi:10.4103/0250-474X.65012
- Liu ZJ, Liu W, Liu L, Xiao C, Wang Y, Jiao JS. Curcumin Protects Neuron against Cerebral Ischemia-Induced Inflammation through Improving PPAR-Gamma Function. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:470975. doi:10.1155/2013/470975
- Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. Feb 2008;22(2):141-8. doi:10.1002/ptr.2295
- Flodin NW. The metabolic roles, pharmacology, and toxicology of lysine. J Am Coll Nutr. Feb 1997;16(1):7-21. doi:10.1080/07315724.1997.10718644
- Griffith RS, Walsh DE, Myrmel KH, Thompson RW, Behforooz A. Success of L-lysine therapy in frequently recurrent herpes simplex infection. Treatment and prophylaxis. Dermatologica. 1987;175(4):183-90.
- Kennedy PG. Herpes simplex virus type 1 and Bell's palsy-a current assessment of the controversy. J Neurovirol. Feb 2010;16(1):1-5. doi:10.3109/13550280903552446
- Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. Oct 25 1979;281(5733):689-90. doi:10.1038/281689a0
- Pu JY, He L, Wu SY, Zhang P, Huang X. [Anti-virus research of triterpenoids in licorice]. Bing Du Xue Bao. Nov 2013;29(6):673-9.
- Sekizawa T, Yanagi K, Itoyama Y. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. Feb 2001;45(1):51-4.
- O'Dell BL, Conley-Harrison J, Besch-Williford C, Browning JD, O'Brien D. Zinc status and peripheral nerve function in guinea pigs. FASEB journal : official publication of the Federation of American Societies for Experimental Biology . Aug 1990;4(11):2919-22. doi:10.1096/fasebj.4.11.2165949
- Ozden F, Turanli A, Acikgoz G, Eroglu C. Clinical success of lysine in association with serumal and salivary presence of HSV-1 in patients with recurrent aphthous ulceration. Journal of experimental and integrative medicine. 2011;1:191-196.
- EBSCO CAM Review Board. Lysine. NYU Langone Medical Center. Updated 6/2011. Accessed 1/3/2013, http://www.med.nyu.edu/content?ChunkIID=21791
- Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, de Vincentiis M. Bell's palsy and autoimmunity. Autoimmun Rev. Dec 2012;12(2):323-8. doi:10.1016/j.autrev.2012.05.008
- Ogawa A, Sando I. Spatial occupancy of vessels and facial nerve in the facial canal. Ann Otol Rhinol Laryngol. Jan-Feb 1982;91(1 Pt 1):14-9. doi:10.1177/000348948209100105
- Sekiya T, Okabe S, Hatayama T, Iwabuchi T, Takiguchi M. [Postoperative facial and vestibular nerve palsy: experimental study of its pathophysiological mechanisms]. No To Shinkei. Feb 1990;42(2):113-9.
- Gantz BJ, Fisch U. Modified transotic approach to the cerebellopontile angle. Arch Otolaryngol. Apr 1983;109(4):252-6. doi:10.1001/archotol.1983.00800180050010
- Kölln K, LaCour J, Pillsbury HC. Netter's Internal Medicine. Second Edition. Copyright 2009, an Imprint of Elsevier. Clinical Key. https://www.clinicalkey.com
- Grewal DS. Bell's Palsy-Tertiary Ischemia: An Etiological Factor in Residual Facial Palsy. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India . Sep 2018;70(3):374-379. doi:10.1007/s12070-018-1381-9
- Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. Apr 1992;31(4):444-8. doi:10.1002/ana.410310417
- Steiner I, Kennedy PG. Herpes simplex virus latent infection in the nervous system. J Neurovirol. Mar 1995;1(1):19-29. doi:10.3109/13550289509111007
- Taylor TJ, Brockman MA, McNamee EE, Knipe DM. Herpes simplex virus. Front Biosci. Mar 1 2002;7:d752-64. doi:10.2741/taylor
- Ordoñez G, Vales O, Pineda B, Rodríguez K, Pane C, Sotelo J. The presence of herpes simplex-1 and varicella zoster viruses is not related with clinical outcome of Bell's Palsy. Virology. Oct 2020;549:85-88. doi:10.1016/j.virol.2020.07.020
- Turriziani O, Falasca F, Maida P, et al. Early collection of saliva specimens from Bell's palsy patients: quantitative analysis of HHV-6, HSV-1, and VZV. Journal of medical virology. Oct 2014;86(10):1752-8. doi:10.1002/jmv.23917
- Bremell D, Hagberg L. Clinical characteristics and cerebrospinal fluid parameters in patients with peripheral facial palsy caused by Lyme neuroborreliosis compared with facial palsy of unknown origin (Bell's palsy). BMC infectious diseases. Aug 10 2011;11:215. doi:10.1186/1471-2334-11-215
- Cooper L, Branagan-Harris M, Tuson R, Nduka C. Lyme disease and Bell's palsy: an epidemiological study of diagnosis and risk in England. The British journal of general practice : the journal of the Royal College of General Practitioners . May 2017;67(658):e329-e335. doi:10.3399/bjgp17X690497
- Pacheco A, Rutler O, Valenzuela I, Feldman D, Eskin B, Allegra JR. Positive Tests for Lyme Disease and Emergency Department Visits for Bell's Palsy Patients. The Journal of emergency medicine. Dec 2020;59(6):820-827. doi:10.1016/j.jemermed.2020.07.038
- Ferri FF. Ferri's Clinical Advisor. "Bell's Palsy". St. Louis, Mosby; 2014.
- Morgan M, Nathwani D. Facial palsy and infection: the unfolding story. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America . Jan 1992;14(1):263-71. doi:10.1093/clinids/14.1.263
- Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. Apr 2021;21(4):450-452. doi:10.1016/s1473-3099(21)00076-1
- Cirillo N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology . Apr 2021;50(4):424-427. doi:10.1111/jop.13165
- Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Human vaccines & immunotherapeutics. Mar 4 2018;14(3):550-564. doi:10.1080/21645515.2017.1415684
- Ho K, Melanson M, Desai JA. Bell palsy in lyme disease-endemic regions of canada: a cautionary case of occult bilateral peripheral facial nerve palsy due to Lyme disease. Cjem. Sep 2012;14(5):321-4. doi:10.2310/8000.2011.110512
- Zulfiqar S, Qureshi A, Dande R, Puri C, Persaud K, Awasthi S. The many manifestations of a single disease: neuroborreliosis. J Community Hosp Intern Med Perspect. Jan 26 2021;11(1):56-59. doi:10.1080/20009666.2020.1831746
- López-Alberola RF. Neuroborreliosis and the pediatric population: a review. Rev Neurol. Apr 10 2006;42 Suppl 3:S91-6.
- Yang A, Dalal V. Bilateral Facial Palsy: A Clinical Approach. Cureus. Apr 25 2021;13(4):e14671. doi:10.7759/cureus.14671
- Kumar M, Acharya S, Vineetha R, Pai KM. Bilateral Bell's palsy in a young female: a rare case report. Med Pharm Rep. Jan 2021;94(1):118-120. doi:10.15386/mpr-1389
- Sekelj A, Đanić D. Acoustic Reflex and House-Brackmann Rating Scale as Prognostic Indicators of Peripheral Facial Palsy in Neuroborreliosis. Acta Clin Croat. Sep 2017;56(3):425-436. doi:10.20471/acc.2017.56.03.09
- Wormser GP, McKenna D, Scavarda C, Karmen C. Outcome of facial palsy from Lyme disease in prospectively followed patients who had received corticosteroids. Diagn Microbiol Infect Dis. Aug 2018;91(4):336-338. doi:10.1016/j.diagmicrobio.2018.03.016
- Jowett N, Gaudin RA, Banks CA, Hadlock TA. Steroid use in Lyme disease-associated facial palsy is associated with worse long-term outcomes. The Laryngoscope. Jun 2017;127(6):1451-1458. doi:10.1002/lary.26273
- Centers for Disease Control and Prevention. Lyme Disease: Treatment. Accessed 6/23/2021, https://www.cdc.gov/lyme/treatment/index.html
- Hirsch AG, Poulsen MN, Nordberg C, et al. Risk Factors and Outcomes of Treatment Delays in Lyme Disease: A Population-Based Retrospective Cohort Study. Front Med (Lausanne). 2020;7:560018. doi:10.3389/fmed.2020.560018
- Rojko T, Bogovič P, Lotrič-Furlan S, et al. Borrelia burgdorferi sensu lato infection in patients with peripheral facial palsy. Ticks Tick Borne Dis. Feb 2019;10(2):398-406. doi:10.1016/j.ttbdis.2018.11.019
- Kindler W, Wolf H, Thier K, Oberndorfer S. Peripheral facial palsy as an initial symptom of Lyme neuroborreliosis in an Austrian endemic area. Wiener klinische Wochenschrift. Nov 2016;128(21-22):837-840. Periphere Facialisparese als Initialsymptom der Lyme Neuroborreliose in einem österreichischen Endemiegebiet. doi:10.1007/s00508-014-0685-3
- CDC. Centers for Disease Control and Prevention: Lyme Disease. Available at https://www.cdc.gov/lyme/index.html . Last updated 05/28/2021. Accessed 06/15/2021. . 2021;
- Cirpaciu D, Goanta CM, Cirpaciu MD. Recurrences of Bell's palsy. J Med Life. 2014;7 Spec No. 3(Spec Iss 3):68-77.
- Skuladottir AT, Bjornsdottir G, Thorleifsson G, et al. A meta-analysis uncovers the first sequence variant conferring risk of Bell's palsy. Sci Rep. Feb 18 2021;11(1):4188. doi:10.1038/s41598-021-82736-w
- Papan C, Kremp L, Weiß C, Petzold A, Schroten H, Tenenbaum T. Infectious causes of peripheral facial nerve palsy in children-a retrospective cohort study with long-term follow-up. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology . Nov 2019;38(11):2177-2184. doi:10.1007/s10096-019-03660-6
- Dahl EH, Mosevoll KA, Cramariuc D, Vedeler CA, Blomberg B. COVID-19 myocarditis and postinfection Bell's palsy. BMJ case reports. Jan 11 2021;14(1)doi:10.1136/bcr-2020-240095
- Psillas G, Antoniades E, Ieridou F, Constantinidis J. Facial nerve palsy in children: A retrospective study of 124 cases. J Paediatr Child Health. Mar 2019;55(3):299-304. doi:10.1111/jpc.14190
- Völter C, Helms J, Weissbrich B, Rieckmann P, Abele-Horn M. Frequent detection of Mycoplasma pneumoniae in Bell's palsy. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery . Aug 2004;261(7):400-4. doi:10.1007/s00405-003-0676-x
- Henkel K, Lange P, Eiffert H, Nau R, Spreer A. Infections in the differential diagnosis of Bell's palsy: a plea for performing CSF analysis. Infection. Apr 2017;45(2):147-155. doi:10.1007/s15010-016-0933-8
- Fuzi J, Spencer S, Seckold E, Damiano S, Meller C. Bell's palsy during pregnancy and the post-partum period: A contemporary management approach. American journal of otolaryngology. Jan 12 2021;42(3):102914. doi:10.1016/j.amjoto.2021.102914
- Aditya V. LMN Facial Palsy in Pregnancy: An Opportunity to Predict Preeclampsia-Report and Review. Case Rep Obstet Gynecol. 2014;2014:626871. doi:10.1155/2014/626871
- Klein A. Peripheral nerve disease in pregnancy. Clin Obstet Gynecol. Jun 2013;56(2):382-8. doi:10.1097/GRF.0b013e31828f260e
- Pourrat O, Neau JP, Pierre F. Bell's palsy in pregnancy: underlying HELLP syndrome or pre-eclampsia? Obstet Med. Sep 2013;6(3):132-133. doi:10.1258/om.2012.110093
- Vogell A, Boelig RC, Skora J, Baxter JK. Bilateral Bell palsy as a presenting sign of preeclampsia. Obstet Gynecol. Aug 2014;124(2 Pt 2 Suppl 1):459-461. doi:10.1097/aog.0000000000000221
- Evangelista V, Gooding MS, Pereira L. Bell's Palsy in Pregnancy. Obstetrical & gynecological survey. Nov 2019;74(11):674-678. doi:10.1097/ogx.0000000000000732
- Lee CC, Su YC, Chien SH, et al. Increased stroke risk in Bell's palsy patients without steroid treatment. European journal of neurology : the official journal of the European Federation of Neurological Societies . Apr 2013;20(4):616-22. doi:10.1111/j.1468-1331.2012.03765.x
- Tseng CC, Hu LY, Liu ME, Yang AC, Shen CC, Tsai SJ. Bidirectional association between Bell's palsy and anxiety disorders: A nationwide population-based retrospective cohort study. J Affect Disord. Jun 2017;215:269-273. doi:10.1016/j.jad.2017.03.051
- Yoo MC, Soh Y, Chon J, et al. Evaluation of Factors Associated With Favorable Outcomes in Adults With Bell Palsy. JAMA Otolaryngol Head Neck Surg. Mar 1 2020;146(3):256-263. doi:10.1001/jamaoto.2019.4312
- Jung SY, Jung J, Byun JY, Park MS, Kim SH, Yeo SG. The effect of metabolic syndrome on Bell's palsy recovery rate. Acta oto-laryngologica. Jul 2018;138(7):670-674. doi:10.1080/00016489.2018.1425902
- Cai Z, Li H, Wang X, et al. Prognostic factors of Bell's palsy and Ramsay Hunt syndrome. Medicine. Jan 2017;96(2):e5898. doi:10.1097/md.0000000000005898
- Kim SY, Oh DJ, Park B, Choi HG. Bell's palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci Rep. Mar 6 2020;10(1):4248. doi:10.1038/s41598-020-61240-7
- Choi SA, Shim HS, Jung JY, et al. Association between recovery from Bell's palsy and body mass index. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery . Jun 2017;42(3):687-692. doi:10.1111/coa.12801
- Kim SY, Lee CH, Lim JS, Kong IG, Sim S, Choi HG. Increased risk of Bell palsy in patient with migraine: A longitudinal follow-up study. Medicine. May 2019;98(21):e15764. doi:10.1097/md.0000000000015764
- Sheu JJ, Keller JJ, Lin HC. Increased risk of cancer after Bell's palsy: a 5-year follow-up study. Journal of neuro-oncology. Nov 2012;110(2):215-20. doi:10.1007/s11060-012-0954-9
- Misulis KE, Galloway GM, et al. Bell's palsy. First Consult. Updated 8/11/2010. Accessed 4/29/2014, www.clinicalkey.com
- Kim YH, Choi IJ, Kim HM, Ban JH, Cho CH, Ahn JH. Bilateral simultaneous facial nerve palsy: clinical analysis in seven cases. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology . Apr 2008;29(3):397-400. doi:10.1097/mao.0b013e3181656998
- Lynch PJ. Bell's palsy diagram. Wikimedia Commons. Updated 9/13/2020. Accessed 6/10/2021, https://commons.wikimedia.org/wiki/File:Bells_palsy_diagram.svg
- Holland NJ, Weiner GM. Recent developments in Bell's palsy. BMJ (Clinical research ed). Sep 4 2004;329(7465):553-7. doi:10.1136/bmj.329.7465.553
- Tiemstra JD, Khatkhate N. Bell's palsy: diagnosis and management. American family physician. Oct 1 2007;76(7):997-1002.
- Fahimi J, Navi BB, Kamel H. Potential misdiagnoses of Bell's palsy in the emergency department. Annals of emergency medicine. Apr 2014;63(4):428-34. doi:10.1016/j.annemergmed.2013.06.022
- Mayo Clinic. Diseases and Conditions. Symptoms and causes. Stroke. Updated 2/9/2021. Accessed 4/16/2021, http://www.mayoclinic.org/diseases-conditions/stroke/basics/definition/CON-20042884
- NIH. U.S. National Library of Medicine. MedlinePlus. Facial paralysis. Updated 4/2/2021. Accessed 4/16/2021, http://www.nlm.nih.gov/medlineplus/ency/article/003028.htm
- Zaidi FH, Gregory-Evans K, Acheson JF, Ferguson V. Familial Bell's palsy in females: a phenotype with a predilection for eyelids and lacrimal gland. Orbit (Amsterdam, Netherlands). Jun 2005;24(2):121-4. doi:10.1080/01676830490916082
- Gilden DH. Bell's Palsy. New England Journal of Medicine. 2004;351(13):1323-1331. doi:10.1056/NEJMcp041120
- Ng JH, Ngo RY. The use of the facial clinimetric evaluation scale as a patient-based grading system in Bell's palsy. The Laryngoscope. May 2013;123(5):1256-60. doi:10.1002/lary.23790
- Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery . Feb 2009;140(2):154-8. doi:10.1016/j.otohns.2008.11.021
- Yen TL, Driscoll CL, Lalwani AK. Significance of House-Brackmann facial nerve grading global score in the setting of differential facial nerve function. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology . Jan 2003;24(1):118-22. doi:10.1097/00129492-200301000-00023
- Lampert L, Wong YJ. Combined antiviral-corticosteroid therapy for Bell palsy yields inconclusive benefit. Journal of the American Dental Association (1939). Jan 2012;143(1):57-8. doi:10.14219/jada.archive.2012.0020
- Cotler HB, Chow RT, Hamblin MR, Carroll J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop Rheumatol. 2015;2(5)doi:10.15406/mojor.2015.02.00068
- Kim SJ, Lee HY. Acute Peripheral Facial Palsy: Recent Guidelines and a Systematic Review of the Literature. J Korean Med Sci. Aug 3 2020;35(30):e245. doi:10.3346/jkms.2020.35.e245
- Alayat MS, Elsodany AM, El Fiky AA. Efficacy of high and low level laser therapy in the treatment of Bell's palsy: a randomized double blind placebo-controlled trial. Lasers in medical science. Jan 2014;29(1):335-42. doi:10.1007/s10103-013-1352-z
- Ordahan B, Karahan AY. Role of low-level laser therapy added to facial expression exercises in patients with idiopathic facial (Bell's) palsy. Lasers in medical science. May 2017;32(4):931-936. doi:10.1007/s10103-017-2195-9
- Colombo F, Marques AM, Carvalho C, et al. The effect of the photobiomodulation in the treatment of Bell's palsy: clinical experience . vol 8211. SPIE BiOS. SPIE; 2012.
- Aghamohamdi D, Fakhari S, Farhoudi M, Farzin H. The Efficacy of Low-Level Laser Therapy in the Treatment of Bell's Palsy in Diabetic Patients. J Lasers Med Sci. Summer 2020;11(3):310-315. doi:10.34172/jlms.2020.52
- Fontana CR, Bagnato VS. Low-level laser therapy in pediatric Bell's palsy: case report in a three-year-old child. Journal of alternative and complementary medicine (New York, NY). Apr 2013;19(4):376-82. doi:10.1089/acm.2011.0531
- Poloni MM, Marques NP, Ribeiro Junior NV, Sperandio FF, Hanemann JAC, de Carli ML. Bell's palsy treated with photobiomodulation in an adolescent: Rare case report and review of the published literature. Int J Paediatr Dent. Nov 2018;28(6):658-662. doi:10.1111/ipd.12424
- Tanganeli JPC, de Oliveira SSI, da Silva T, Fernandes KPS, Motta LJ, Bussadori SK. Complete and Fast Recovery from Idiopathic Facial Paralysis Using Laser-Photobiomodulation. Case Rep Dent. 2020;2020:9867693. doi:10.1155/2020/9867693
- Javaherian M, Attarbashi Moghaddam B, Bashardoust Tajali S, Dabbaghipour N. Efficacy of low-level laser therapy on management of Bell's palsy: a systematic review. Lasers in medical science. Aug 2020;35(6):1245-1252. doi:10.1007/s10103-020-02996-2
- Husseman J, Mehta RP. Management of synkinesis. Facial plastic surgery : FPS. May 2008;24(2):242-9. doi:10.1055/s-2008-1075840
- Mayo Clinic. Diseases and Conditions. Symptoms and causes. Bell's palsy. Updated 4/2/2020. Accessed 4/16/2021, http://www.mayoclinic.org/diseases-conditions/bells-palsy/basics/definition/CON-20020529
- Wiener A, Touloei K, Glick BP. A Novel Long-term Therapy of Facial Synkinesis with Botulinum Neurotoxins Type A and Fillers. The Journal of clinical and aesthetic dermatology. Mar 2011;4(3):45-9.
- Kim J. Contralateral botulinum toxin injection to improve facial asymmetry after acute facial paralysis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology . Feb 2013;34(2):319-24. doi:10.1097/mao.0b013e31827c9f58
- Holland NJ, Bernstein JM, Hamilton JW. Hyperbaric oxygen therapy for Bell's palsy. Cochrane Database of Systematic Reviews. 2012;(2)doi:10.1002/14651858.CD007288.pub2
- Racic G, Denoble PJ, Sprem N, Bojic L, Bota B. Hyperbaric oxygen as a therapy of Bell's palsy. Undersea Hyperb Med. 1997;24(1):35-8.
- Kinishi M, Hosomi H, Amatsu M. [Conservative treatment of Bell's palsy--high dose steroid infusion with low-molecular dextran]. Nihon Jibiinkoka Gakkai kaiho. May 1989;92(5):694-702. doi:10.3950/jibiinkoka.92.694
- Kinishi M, Amatsu M, Hosomi H. Conservative treatment of Bell's palsy with steroids and dextran-pentoxiphylline combined therapy. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery . 1991;248(3):147-9. doi:10.1007/bf00178925
- Sittel C, Stennert E. [Antiphlogisitc-rheologic infusion therapy of acute idiopathic facial paralysis. Experiences and results of 344 cases]. Hno. Aug 2000;48(8):573-82. Antiphlogistisch-rheologische Infusionstherapie der akuten idiopathischen Fazialisparese. Erfahrungen und Ergebnisse aus 344 Fällen. doi:10.1007/s001060050619
- Sittel C, Sittel A, Guntinas-Lichius O, Eckel HE, Stennert E. Bell's palsy: a 10-year experience with antiphlogistic-rheologic infusion therapy. Am J Otol. May 2000;21(3):425-32. doi:10.1016/s0196-0709(00)80055-1
- Su Y, Dong X, Liu J, Hu Y, Chen J. Nerve growth factor for Bell's palsy: A meta-analysis. Experimental and therapeutic medicine. Feb 2015;9(2):501-506. doi:10.3892/etm.2014.2100
- Inglis VB. Requirement of arginine for the replication of herpes virus. The Journal of general virology. Jul 1968;3(1):9-17. doi:10.1099/0022-1317-3-1-9
- Tankersley RW, Jr. AMINO ACID REQUIREMENTS OF HERPES SIMPLEX VIRUS IN HUMAN CELLS. J Bacteriol. Mar 1964;87(3):609-13. doi:10.1128/jb.87.3.609-613.1964
- Zhang R, Wu T, Wang R, Wang D, Liu Q. Compare the efficacy of acupuncture with drugs in the treatment of Bell's palsy: A systematic review and meta-analysis of RCTs. Medicine. May 2019;98(19):e15566. doi:10.1097/md.0000000000015566
- Li P, Qiu T, Qin C. Efficacy of Acupuncture for Bell's Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One . 2015;10(5):e0121880. doi:10.1371/journal.pone.0121880
- Bian Y, He X, Hu S, et al. Functional Connectivity Modulation by Acupuncture in Patients with Bell's Palsy. Evidence-based complementary and alternative medicine : eCAM. 2016;2016:5928758. doi:10.1155/2016/5928758
- Wu H, Kan H, Li C, et al. Effect of Acupuncture on Functional Connectivity of Anterior Cingulate Cortex for Bell's Palsy Patients with Different Clinical Duration. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:646872. doi:10.1155/2015/646872
- Wang WH, Jiang RW, Liu NC. Electroacupuncture Is Effective for Peripheral Facial Paralysis: A Meta-Analysis. Evidence-based complementary and alternative medicine : eCAM. 2020;2020:5419407. doi:10.1155/2020/5419407
- Xiao X, Zheng Q, Shi Y, et al. Association of Patients' Characteristics with Acupuncture Treatment Outcomes in Treating Bell's Palsy: Results from a Randomised Controlled Trial. Evidence-based complementary and alternative medicine : eCAM. 2019;2019:6073484. doi:10.1155/2019/6073484
- Pourmomeny AA, Asadi S. Management of synkinesis and asymmetry in facial nerve palsy: a review article. Iran J Otorhinolaryngol. Oct 2014;26(77):251-6.
- Baba S, Kondo K, Yoshitomi A, Kanemaru A, Nakaya M, Yamasoba T. Efficacy of Mirror Biofeedback Rehabilitation on Synkinesis in Acute Stage Facial Palsy in Children. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology . Mar 19 2021;doi:10.1097/mao.0000000000003144
- Pourmomeny AA, Asadi S, Cheatsaz A. Management of Facial Synkinesis with a Combination of BTX-A and Biofeedback: A Randomized Trial. Iran J Otorhinolaryngol. Nov 2015;27(83):409-15.
- Mandrini S, Comelli M, Dall'angelo A, et al. Long-term facial improvement after repeated BoNT-A injections and mirror biofeedback exercises for chronic facial synkinesis: a case-series study. European journal of physical and rehabilitation medicine. Dec 2016;52(6):810-818.
- Paolucci T, Cardarola A, Colonnelli P, et al. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. European journal of physical and rehabilitation medicine. Feb 2020;56(1):58-67. doi:10.23736/s1973-9087.19.05757-5
- Di Stadio A, Gambacorta V, Ralli M, et al. Facial taping as biofeedback to improve the outcomes of physical rehab in Bell's palsy: preliminary results of a randomized case-control study. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery . May 2021;278(5):1693-1698. doi:10.1007/s00405-020-06193-3
- Kasahara T, Ikeda S, Sugimoto A, et al. Efficacy of Tape Feedback Therapy on Synkinesis Following Severe Peripheral Facial Nerve Palsy. The Tokai journal of experimental and clinical medicine. Sep 20 2017;42(3):139-142.
- Beurskens CH, Heymans PG. Mime therapy improves facial symmetry in people with long-term facial nerve paresis: a randomised controlled trial. Aust J Physiother. 2006;52(3):177-83. doi:10.1016/s0004-9514(06)70026-5
- Teixeira LJ, Valbuza JS, Prado GF. Physical therapy for Bell's palsy (idiopathic facial paralysis). The Cochrane database of systematic reviews. Dec 7 2011;(12):Cd006283. doi:10.1002/14651858.CD006283.pub3
- Barbara M, Antonini G, Vestri A, Volpini L, Monini S. Role of Kabat physical rehabilitation in Bell's palsy: a randomized trial. Acta oto-laryngologica. 2010;130(1):167-72. doi:10.3109/00016480902882469
- Agostini F, Mangone M, Santilli V, et al. Idiopathic facial palsy: umbrella review of systematic reviews and meta-analyses. Journal of biological regulators and homeostatic agents. Jul-Aug 2020;34(4):1245-1255. doi:10.23812/20-339-a
- Tanaka H. [Old or new medicine? Vitamin B12 and peripheral nerve neuropathy]. Brain Nerve. Sep 2013;65(9):1077-82.
- Rizzo G, Laganà AS. Chapter 6 - A review of vitamin B12. In: Patel VB, ed. Molecular Nutrition. Academic Press; 2020:105-129.
- Wang LL, Guan L, Hao PL, Du JL, Zhang MX. Acupuncture and vitamin B12 injection for Bell's palsy: no high-quality evidence exists. Neural Regen Res. May 2015;10(5):808-13. doi:10.4103/1673-5374.156987
- Sickels M. Treatment options for patients with Bell's palsy. American family physician. Aug 1 2008;78(3):316, 319; author reply 319-20.
- Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. Apr 24 2006;397(3):219-23. doi:10.1016/j.neulet.2005.12.013
- Sima AA, Calvani M, Mehra M, Amato A. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. Jan 2005;28(1):89-94. doi:10.2337/diacare.28.1.89
- Kime CE. Bell's palsy: a new syndrome associated with treatment by nicotinic acid; a guide to adequate medical therapy. AMA Arch Otolaryngol. Jul 1958;68(1):28-32. doi:10.1001/archotol.1958.00730020032004
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochemical Society transactions. Oct 15 2017;45(5):1105-1115. doi:10.1042/BST20160474
- Chamani S, Bianconi V, Tasbandi A, et al. Resolution of Inflammation in Neurodegenerative Diseases: The Role of Resolvins. Mediators Inflamm. 2020;2020:3267172. doi:10.1155/2020/3267172
- Gladman SJ, Huang W, Lim SN, et al. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous ω-3 polyunsaturated fatty acids. J Neurosci. Jan 11 2012;32(2):563-71. doi:10.1523/jneurosci.3371-11.2012
- Ochi E, Tsuchiya Y, Yanagimoto K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. Journal of the International Society of Sports Nutrition. 2017;14:23. doi:10.1186/s12970-017-0176-9
- Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology. Jun 13 2017;88(24):2294-2301. doi:10.1212/wnl.0000000000004033
- Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, Perry G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics . Jul 2019;16(3):666-674. doi:10.1007/s13311-019-00767-8
- Lejri I, Grimm A, Eckert A. Ginkgo biloba extract increases neurite outgrowth and activates the Akt/mTOR pathway. PLoS One. 2019;14(12):e0225761. doi:10.1371/journal.pone.0225761
- Yin JJ, He Y, An J, et al. Dynamic Balance of Microglia and Astrocytes Involved in the Remyelinating Effect of Ginkgolide B. Frontiers in cellular neuroscience. 2019;13:572. doi:10.3389/fncel.2019.00572
- Mohammed NA, Abdou HM, Tass MA, Alfwuaires M, Abdel-Moneim AM, Essawy AE. Oral Supplements of <i>Ginkgo biloba</i> Extract Alleviate Neuroinflammation, Oxidative Impairments and Neurotoxicity in Rotenone-Induced Parkinsonian Rats. Current pharmaceutical biotechnology. 2020;21(12):1259-1268. doi:10.2174/1389201021666200320135849
- Gargouri B, Carstensen J, Bhatia HS, Huell M, Dietz GPH, Fiebich BL. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine. May 15 2018;44:45-55. doi:10.1016/j.phymed.2018.04.009
- Al-Adwani DG, Renno WM, Orabi KY. Neurotherapeutic effects of Ginkgo biloba extract and its terpene trilactone, ginkgolide B, on sciatic crush injury model: A new evidence. PLoS One. 2019;14(12):e0226626. doi:10.1371/journal.pone.0226626
- Cho HK, Kim S, Lee EJ, Kee C. Neuroprotective Effect of Ginkgo Biloba Extract Against Hypoxic Retinal Ganglion Cell Degeneration In Vitro and In Vivo. Journal of medicinal food. Aug 2019;22(8):771-778. doi:10.1089/jmf.2018.4350
- Kaur S, Sharma N, Nehru B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacol. Feb 2018;26(1):87-104. doi:10.1007/s10787-017-0396-2
- Zhou X, Qi Y, Chen T. Long-term pre-treatment of antioxidant Ginkgo biloba extract EGb-761 attenuates cerebral-ischemia-induced neuronal damage in aged mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie . Jan 2017;85:256-263. doi:10.1016/j.biopha.2016.11.013
- Huang WL, Ma YX, Fan YB, et al. Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus after exposure to acrylamide. Neural Regen Res. Aug 2017;12(8):1287-1293. doi:10.4103/1673-5374.213548
- Borenstein R, Hanson BA, Markosyan RM, et al. Ginkgolic acid inhibits fusion of enveloped viruses. Sci Rep. Mar 16 2020;10(1):4746. doi:10.1038/s41598-020-61700-0
- Potter PE. Curcumin: a natural substance with potential efficacy in Alzheimer's disease. J Exp Pharmacol. 2013;5:23-31. doi:10.2147/jep.S26803
- Scapagnini G, Colombrita C, Amadio M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. Mar-Apr 2006;8(3-4):395-403. doi:10.1089/ars.2006.8.395
- Huang HC, Lin CJ, Liu WJ, Jiang RR, Jiang ZF. Dual effects of curcumin on neuronal oxidative stress in the presence of Cu(II). Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association . Jul 2011;49(7):1578-83. doi:10.1016/j.fct.2011.04.004
- Cheng CY, Barro L, Tsai ST, et al. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. International journal of molecular sciences. Mar 16 2021;22(6)doi:10.3390/ijms22063037
- Lai SW, Chen JH, Lin HY, et al. Regulatory Effects of Neuroinflammatory Responses Through Brain-Derived Neurotrophic Factor Signaling in Microglial Cells. Molecular neurobiology. Sep 2018;55(9):7487-7499. doi:10.1007/s12035-018-0933-z
- Mao L, Hochstetter D, Yao L, et al. Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. International journal of molecular sciences. Oct 13 2019;20(20)doi:10.3390/ijms20205081
- Zhou J, Mao L, Xu P, Wang Y. Effects of (-)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients. Nov 5 2018;10(11)doi:10.3390/nu10111681
- Wang J, Li P, Qin T, Sun D, Zhao X, Zhang B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain and behavior. Jun 2020;10(6):e01633. doi:10.1002/brb3.1633
- Cheng-Chung Wei J, Huang HC, Chen WJ, Huang CN, Peng CH, Lin CL. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia. European journal of pharmacology. Jan 5 2016;770:16-24. doi:10.1016/j.ejphar.2015.11.048
- Tan SW, Israf Ali DAB, Khaza'ai H, Wong JW, Vidyadaran S. Cellular uptake and anti-inflammatory effects of palm oil-derived delta (δ)-tocotrienol in microglia. Cell Immunol. Nov 2020;357:104200. doi:10.1016/j.cellimm.2020.104200
- Manosso LM, Camargo A, Dafre AL, Rodrigues ALS. Vitamin E for the management of major depressive disorder: possible role of the anti-inflammatory and antioxidant systems. Nutritional neuroscience. Dec 14 2020:1-15. doi:10.1080/1028415x.2020.1853417
- Ambrogini P, Torquato P, Bartolini D, et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim Biophys Acta Mol Basis Dis. Jun 1 2019;1865(6):1098-1112. doi:10.1016/j.bbadis.2019.01.026
- Betti M, Minelli A, Ambrogini P, et al. Dietary supplementation with α-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic Res . Oct 2011;45(10):1136-42. doi:10.3109/10715762.2011.597750
- Ambrogini P, Minelli A, Galati C, et al. Post-seizure α-tocopherol treatment decreases neuroinflammation and neuronal degeneration induced by status epilepticus in rat hippocampus. Molecular neurobiology. Aug 2014;50(1):246-56. doi:10.1007/s12035-014-8648-2
- Sheridan PA, Beck MA. The immune response to herpes simplex virus encephalitis in mice is modulated by dietary vitamin E. J Nutr. Jan 2008;138(1):130-7. doi:10.1093/jn/138.1.130
- Tutelyan VA, Makhova AA, Pogozheva AV, Shikh EV, Elizarova EV, Khotimchenko SA. [Lipoic acid: physiological role and prospects for clinical application]. Voprosy pitaniia. 2019;88(4):6-11. doi:10.24411/0042-8833-2019-10035
- Salehi B, Berkay Yılmaz Y, Antika G, et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules. Aug 9 2019;9(8)doi:10.3390/biom9080356
- Nguyen H, Gupta V. Alpha-Lipoic Acid. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Ajith TA. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin Exp Pharmacol Physiol. Dec 2020;47(12):1883-1890. doi:10.1111/1440-1681.13373
- Chukanova EI, Chukanova AS. [Alpha-lipoic acid in the treatment of diabetic polyneuropathy]. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118(1):103-109. Al'fa-lipoevaia kislota v lechenii diabeticheskoĭ polineĭropatii. doi:10.17116/jnevro201811811103-109
- Richard SA. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediators Inflamm. 2021;2021:6699560. doi:10.1155/2021/6699560
- Sun ZG, Zhao TT, Lu N, Yang YA, Zhu HL. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini Rev Med Chem. 2019;19(10):826-832. doi:10.2174/1389557519666190119111125
- Kawahara M, Tanaka KI, Kato-Negishi M. Zinc, Carnosine, and Neurodegenerative Diseases. Nutrients. Jan 29 2018;10(2)doi:10.3390/nu10020147
- Kumar V, Kumar A, Singh K, Avasthi K, Kim JJ. Neurobiology of zinc and its role in neurogenesis. European journal of nutrition. Feb 2021;60(1):55-64. doi:10.1007/s00394-020-02454-3
- Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv Nutr. Jul 1 2019;10(4):696-710. doi:10.1093/advances/nmz013
- Mailoo VJ, Rampes S. Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence. Integrative medicine (Encinitas, Calif). Jun 2017;16(3):42-46.
- Paterson RR. Ganoderma - a therapeutic fungal biofactory. Phytochemistry. Sep 2006;67(18):1985-2001. doi:10.1016/j.phytochem.2006.07.004
- Kim YS, Eo SK, Oh KW, Lee C, Han SS. Antiherpetic activities of acidic protein bound polysacchride isolated from Ganoderma lucidum alone and in combinations with interferons. J Ethnopharmacol. Oct 2000;72(3):451-8. doi:10.1016/s0378-8741(00)00263-4
- Liu J, Yang F, Ye LB, et al. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Ethnopharmacol. Dec 2004;95(2-3):265-72. doi:10.1016/j.jep.2004.07.010
- Li Z, Liu J, Zhao Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Biochem Mol Biol. Jan 31 2005;38(1):34-40. doi:10.5483/bmbrep.2005.38.1.034
- Lu J, He R, Sun P, Zhang F, Linhardt RJ, Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. International journal of biological macromolecules. May 1 2020;150:765-774. doi:10.1016/j.ijbiomac.2020.02.035
- Hijikata Y, Yamada S. Effect of Ganoderma lucidum on postherpetic neuralgia. The American journal of Chinese medicine. 1998;26(3-4):375-81. doi:10.1142/s0192415x98000415
- Hijikata Y, Yamada S, Yasuhara A. Herbal mixtures containing the mushroom Ganoderma lucidum improve recovery time in patients with herpes genitalis and labialis. J Altern Complement Med. Nov 2007;13(9):985-7. doi:10.1089/acm.2006.6297
- Hijikata Y, Yasuhara A, Sahashi Y. Effect of an herbal formula containing Ganoderma lucidum on reduction of herpes zoster pain: a pilot clinical trial. Am J Chin Med. 2005;33(4):517-23. doi:10.1142/s0192415x05003120
- Yarnell E, Abascal K, Rountree R. Herbs for Herpes Simplex Infections. Alternative and Complementary Therapies. 04/01 2009;15:69-74. doi:10.1089/act.2009.15203
- Mazzanti G, Battinelli L, Pompeo C, et al. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat Prod Res. 2008;22(16):1433-40. doi:10.1080/14786410802075939
- Astani A, Reichling J, Schnitzler P. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy. 2012;58(1):70-7. doi:10.1159/000335590
- Schnitzler P, Schuhmacher A, Astani A, Reichling J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine : international journal of phytotherapy and phytopharmacology . Sep 2008;15(9):734-40. doi:10.1016/j.phymed.2008.04.018
- Geuenich S, Goffinet C, Venzke S, et al. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology. Mar 20 2008;5:27. doi:10.1186/1742-4690-5-27
- Nolkemper S, Reichling J, Stintzing FC, Carle R, Schnitzler P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. Dec 2006;72(15):1378-82. doi:10.1055/s-2006-951719
- Allahverdiyev A, Duran N, Ozguven M, Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine : international journal of phytotherapy and phytopharmacology . Nov 2004;11(7-8):657-61. doi:10.1016/j.phymed.2003.07.014
- Dimitrova Z, Dimov B, Manolova N, Pancheva S, Ilieva D, Shishkov S. Antiherpes effect of Melissa officinalis L. extracts. Acta Microbiol Bulg. 1993;29:65-72.
- Koytchev R, Alken RG, Dundarov S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine : international journal of phytotherapy and phytopharmacology . Oct 1999;6(4):225-30. doi:10.1016/s0944-7113(99)80013-0
- Wölbling RH, Leonhardt K. Local therapy of herpes simplex with dried extract from Melissa officinalis. Phytomedicine : international journal of phytotherapy and phytopharmacology . Jun 1994;1(1):25-31. doi:10.1016/s0944-7113(11)80019-x
- Bayat M, Azami Tameh A, Hossein Ghahremani M, et al. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences . Oct 3 2012;20(1):42. doi:10.1186/2008-2231-20-42
- Hosseini SR, Kaka G, Joghataei MT, et al. Coadministration of Dexamethasone and Melissa officinalis Has Neuroprotective Effects in Rat Animal Model with Spinal Cord Injury. Cell J. Apr-Jun 2017;19(1):102-116. doi:10.22074/cellj.2016.4868
- López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. Nov 2009;34(11):1955-61. doi:10.1007/s11064-009-9981-0
- Soodi M, Dashti A, Hajimehdipoor H, Akbari S, Ataei N. Melissa officinalis Acidic Fraction Protects Cultured Cerebellar Granule Neurons Against Beta Amyloid-Induced Apoptosis and Oxidative Stress. Cell J. Winter 2017;18(4):556-564. doi:10.22074/cellj.2016.4722
- NIH. U.S. National Library of Medicine. MedlinePlus. Propolis. Updated 3/24/2021. Accessed 4/16/2021, https://medlineplus.gov/druginfo/natural/390.html
- Almuhayawi MS. Propolis as a novel antibacterial agent. Saudi J Biol Sci. Nov 2020;27(11):3079-3086. doi:10.1016/j.sjbs.2020.09.016
- Demir S, Atayoglu AT, Galeotti F, et al. Antiviral activity of different extracts of standardized propolis preparations against HSV. Antiviral therapy. Feb 23 2021;doi:10.3851/imp3383
- Schnitzler P, Neuner A, Nolkemper S, et al. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. Jan 2010;24 Suppl 1:S20-8. doi:10.1002/ptr.2868
- Münstedt K. Bee products and the treatment of blister-like lesions around the mouth, skin and genitalia caused by herpes viruses-A systematic review. Complement Ther Med. Apr 2019;43:81-84. doi:10.1016/j.ctim.2019.01.014
- Sung SH, Choi GH, Lee NW, Shin BC. External Use of Propolis for Oral, Skin, and Genital Diseases: A Systematic Review and Meta-Analysis. Evidence-based complementary and alternative medicine : eCAM. 2017;2017:8025752. doi:10.1155/2017/8025752
- Jautová J, Zelenková H, Drotarová K, Nejdková A, Grünwaldová B, Hladiková M. Lip creams with propolis special extract GH 2002 0.5% versus aciclovir 5.0% for herpes labialis (vesicular stage) : Randomized, controlled double-blind study. Wien Med Wochenschr. May 2019;169(7-8):193-201. Lippencreme mit 0,5 % Propolis-Spezialextrakt GH 2002 versus 5 % Aciclovir bei Herpes labialis (Bläschenstadium) : Randomisierte, kontrollierte Doppelblindstudie. doi:10.1007/s10354-018-0667-6
- Arenberger P, Arenbergerova M, Hladíková M, Holcova S, Ottillinger B. Comparative Study with a Lip Balm Containing 0.5% Propolis Special Extract GH 2002 versus 5% Aciclovir Cream in Patients with Herpes Labialis in the Papular/Erythematous Stage: A Single-blind, Randomized, Two-arm Study. Curr Ther Res Clin Exp. 2018;88:1-7. doi:10.1016/j.curtheres.2017.10.004
- Luthuli S, Wu S, Cheng Y, Zheng X, Wu M, Tong H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar Drugs. Aug 21 2019;17(9)doi:10.3390/md17090487
- Krylova NV, Ermakova SP, Lavrov VF, et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar Drugs. Apr 22 2020;18(4)doi:10.3390/md18040224
- Sinha S, Astani A, Ghosh T, Schnitzler P, Ray B. Polysaccharides from Sargassum tenerrimum: structural features, chemical modification and anti-viral activity. Phytochemistry. Feb 2010;71(2-3):235-42. doi:10.1016/j.phytochem.2009.10.014
- Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. International immunopharmacology. Jan 2008;8(1):109-16. doi:10.1016/j.intimp.2007.10.017
- Tsubura S, Suzuki A. Case report using 4% fucoidan cream for recurrent oral herpes labialis: patient symptoms markedly improved in terms of time to healing and time to loss of discomfort. Dent Open J. 2017;4(2):19-23. doi:10.17140/DOJ-4-135
- Carrera I, Martínez O, Cacabelos R. Neuroprotection with Natural Antioxidants and Nutraceuticals in the Context of Brain Cell Degeneration: The Epigenetic Connection. Current topics in medicinal chemistry. 2019;19(32):2999-3011. doi:10.2174/1568026619666191202155738
- Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur J Microbiol Immunol (Bp). Oct 3 2019;9(3):73-79. doi:10.1556/1886.2019.00016
- Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Reviews in medical virology. Mar 2019;29(2):e2032. doi:10.1002/rmv.2032
- Farghali M, Ruga S, Morsanuto V, Uberti F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain sciences. Sep 22 2020;10(9)doi:10.3390/brainsci10090660
- Airavaara M, Voutilainen MH, Wang Y, Hoffer B. Neurorestoration. Parkinsonism Relat Disord. Jan 2012;18 Suppl 1(0 1):S143-6. doi:10.1016/s1353-8020(11)70045-1
- Patel S, Vajdy M. Induction of cellular and molecular immunomodulatory pathways by vitamin A and flavonoids. Expert Opin Biol Ther. 2015;15(10):1411-28. doi:10.1517/14712598.2015.1066331
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. TheScientificWorldJournal. 2013;2013:162750. doi:10.1155/2013/162750
- Spagnuolo C, Napolitano M, Tedesco I, Moccia S, Milito A, Russo GL. Neuroprotective Role of Natural Polyphenols. Current topics in medicinal chemistry. 2016;16(17):1943-50. doi:10.2174/1568026616666160204122449
- Rice MW, Pandya JD, Shear DA. Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Frontiers in neurology. 2019;10:875. doi:10.3389/fneur.2019.00875
